Use of early tartaric acid seeding to improve acid retention and sensory characteristics in Petit Verdot during aging (2020)
Kirsty Harmon
Blenheim Vineyards
Summary
Petit Verdot is a relatively popular red grape variety in Virginia, appreciated for its color, tannin, and concentration of flavor and aroma. However, Petit Verdot can also pose challenges in the cellar due to its propensity to complete fermentation with high pH values, leading to fairly large tartaric acid additions. Winemakers debate the timing and amount of acid addition to achieve a microbially stable wine that is still balanced on the palate. The purpose of this experiment was to determine the chemical and sensory effects of a large (4 g/L) addition of tartaric acid to Petit Verdot prior to fermentation.The acidulated wine completed malolactic fermentation with an average pH of 3.64 compared to 4.1 in the untreated wine. Acidulated wine had lower volatile acidity and lower color. However, this wine also received lower scores for volume/body in a sensory test. Acidulation is necessary at times, but the question remains how to know how much acid to add and when.
Introduction
Petit Verdot is a native wine grape variety from Southern France, and a minor player in Bordeaux blends1,2, but often takes center stage in Virginia3. It is the third most common red variety grown in Virginia4, bottled both as a varietal wine and also as a notable contributor to Meritage blends. Petit Verdot provides power, color, tannin, and perception of ripeness, and often fills the same space on the tasting bar as Cabernet Sauvignon in other regions3. In the vineyard, Petit Verdot has small berries and loose clusters, allowing it to fully ripen with less rot our humid climate.1 However, in the winery, Petit Verdot often finishes fermentation with pH values above 4.0, leading to potential for microbial spoilage during long aging in the cellar.
The likely cause of high pH in finished Petit Verdot is excess potassium in the grapes. Potassium in grapes is a function of cultivar, vintage, rootstock, and farming techniques. Grape juice generally has higher potassium than finished wine, with levels as high at 1800-3600 mg/L in high potassium Australian Shiraz5, but lower levels in other regions, such as Bordeaux6. Throughout fermentation, potassium is released and complexes with tartaric acid to form potassium bitartrate (KHT). When in excess, KHT can form crystals and precipitate, leaving the finished wine with higher pH and lower acidity. Though some interventions are possible in the vineyard, some variety/soil combinations will inevitably lead to high potassium juice with the potential to produce high pH wines.
When making red wine from high potassium fruit, the most commonly recommended approach is to use large additions of tartaric acid, however the magnitude and timing of additions can impact the resulting chemical and sensory characters of the wine. High potassium must is a common problem in Australia, and the AWRI recommends acidulating to a pH<3.5 as early as possible (before fermentation) as long as the TA will be <7.5 g/L after the addition. If the TA will be more than 7.5 g/L, they recommend adjusting more, to a pH of 3.4 to account for greater precipitation of KHT5. These adjustments may lead to large additions of tartaric acid. Gardiner (2016)7 outlines experiments with high potassium, high pH Cabernet Sauvignon and Merlot from Pennsylvania, each with over 1600 mg/L potassium, to which 2, 4, and 6 g/L of tartaric acid were added at the beginning of fermentation. In these trials, addition of 5 g/L tartaric was needed in Cabernet Sauvignon and 4 g/L was needed for Merlot to achieve a pH less than 3.7. They noted differences in color with lower pH, but also potentially negative sourness in the higher addition wines.
Addition of very large quantities of tartaric acid prior to fermentation can be daunting; what if the tartaric acid doesn’t precipitate? Gardiner7 recommends testing potassium to determine the amount of tartaric acid needed, however potassium testing is beyond the scope of most small to medium sized wineries and no clear guidelines are given for tartaric additions based on potassium levels. The other approach is to add a lesser amount initially, then more later. This, however, allows for the possibility of microbial spoilage and potential for loss of color prior to stabilization. The purpose of this experiment was to determine the chemical and sensory effects of a large (4 g/L) addition of tartaric acid to Petit Verdot prior to fermentation.
Methods
Grapes were hand harvested on 10/17 and chilled overnight then destemmed and loaded into four macrobins with the addition of 50 mg/L SO2 (as a KMBS addition). Just prior to inoculation, juice samples were taken for general analysis (Brix, pH, TA) as well as an acid trial. Grapes were inoculated with 15g/hL EC1118 yeast the day after processing. After two days of fermentation, 4 g/L tartaric acid was added to the treatment bin only. Bins were punched down twice daily throughout the fermentation until Brix measured lower than -1.5°. Fermentations were monitored for Brix depletion and temperature each day following cap management. Once negative Brix had been reached, fermentations were pressed with free run and press fractions combined. Wine was inoculated with Scott Labs MBR process (0.01 g/L), allowed to settle for 2 weeks, then transferred to barrel for malolactic fermentation in comparable barrels. SO2 (100 mg/L) was added two weeks after malic acid tested 0.05 g/L or less by enzymatic analysis.
Sensory analysis was completed by a panel of 25 wine producers. Due to restrictions put in place during COVID-19, sensory analysis was completed using shipped samples. Each wine producer received three wines in identical bottles, filled on the same day, each coded with random numbers. Two of the bottles contained the same wine while the third bottle contained the different wine. Participants were asked to identify which wine was different (a triangle test). There were four tasting groups with the unique wine in the triangle test balanced among the groups. Participants were then asked to score each wine on a scale of 0 to 10 for fruit intensity, Petit Verdot varietal character, acidity, astringency, and volume/body. Results for the triangle test were analyzed using a one-tailed Z test. Descriptive scores were analyzed using repeated measures ANOVA. Scores for descriptive characteristics were analyzed only from participants who were able to distinguish the two wines (those who answered correctly in the triangle test).
Results
This lot of Petit Verdot was harvested with a pH of 3.36, well within a good range for red fruit in Virginia (Table 1), however, without tartaric acid addition, the pH had risen to 3.7 by the end of alcoholic fermentation. By the end of malolactic fermentation, this value was above 4.0 (Table 2). Acid addition had no effect on fermentation kinetics (Figure 1). The titratable acidity had a notable drop coincident with rising pH levels, indicating there was ample opportunity for acid addition without adverse sensory consequences. The treated lot completed fermentation with a pH of 3.41 and completed malolactic fermentation with a pH averaging 3.65.
Addition of 4 g/L tartaric acid at the beginning of fermentation had several other notable effects on wine chemistry. Early tartaric acid addition appears to have had a notable effect on Potassium, with decreases in the aged wine of almost 700 mg/L compared to control (Table 3). Despite the large addition of tartaric acid, the treatment lot only had 1.2 g/L additional titratable acidity by the end of the aging period (Table 4). The consequent pH shift accomplished by an early addition of tartaric acid meant that rather than aging with an average of 0.32 mg/L molecular SO2, the treatment lot aged with an average of 0.71 mg/L molecular SO2. The volatile acidity in the treatment lot was 0.13 g/L lower than the control lot. pH also has a marked effect on the expression of color (Figure 3).
One drawback of this approach is that it is difficult to know at the beginning of fermentation how much acid is needed to achieve a final target pH. Several authors8,9 have developed equations for the prediction of final pH given various juice components, however many of the measures needed to use these equations are beyond the reach of most small to medium sized wineries and most predictions are only approximate. A practical approach is to perform an acid trial (Appendix A) where standard amounts of tartaric acid are added to the juice with the change in pH measured. However, these trials are limited in predictive value at the juice stages due to ongoing leaching of potassium from the skins of grapes during fermentation. In this experiment, an acid trial predicted only 1 g/L of tartaric acid would be needed to lower juice pH to a pH of 3.23, however the addition of 4 g/L was needed for this effect (Table 5). A later acid trial predicted that 2.25 g/L tartaric acid would be needed to bring the control wine to the same pH as the treatment wine. An addition of this magnitude would likely have serious adverse sensory impacts, and may not take into account the loss of tartaric acid due to KHT precipitation. Despite large early addition of tartaric acid, the control wine completed aging with nearly the same amount as the control (Table 3).
In a triangle test of acidulated vs. non-acidulated wines, 13 out of 25 respondents were able to distinguish which wine was different, indicating the wines were significantly different (Z=1.77, p=0.04). The acidulated wine had significantly higher scores for acidity and significantly lower scores for volume/body. There were no significant differences in scores for fruit intensity, Petit Verdot varietal character, or astringency (Table 6).
Table 1: Fruit chemistry at harvest (in-house data)

Table 2: pH and TA of two treatments of Petit Verdot from fruit through aging (in-house data, ICV labs)
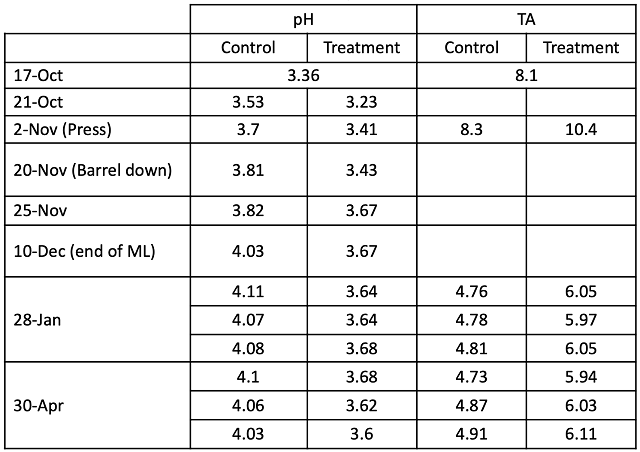
Figure 1: Fermentation kinetics for two treatments of Petit Verdot (in-house data)
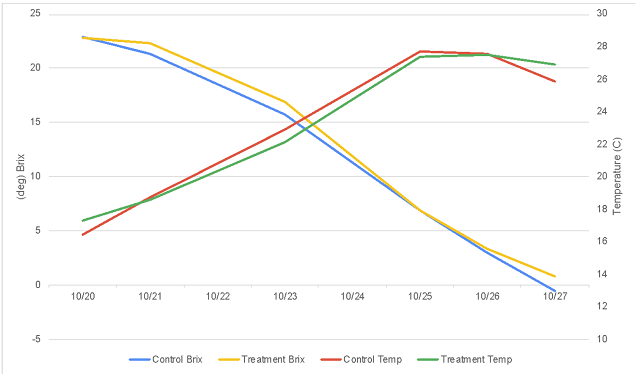
Figure 2: Progression of acidity during fermentation and aging in two treatments of Petit Verdot (in-house lab, ICV)
Table 3: Potassium and tartaric acid after aging (ICV Labs)
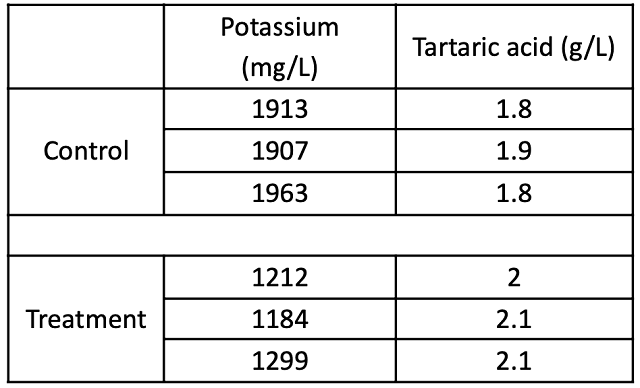
Table 4: Chemistry for three barrels of two treatments at two time points (ICV labs)
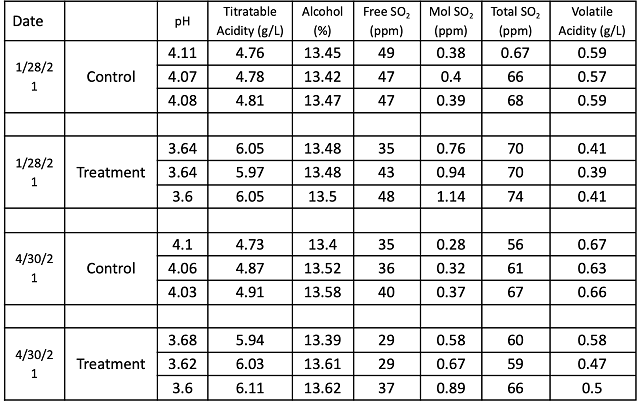
Figure 3: Color metrics for two treatments of Petit Verdot (ICV labs)
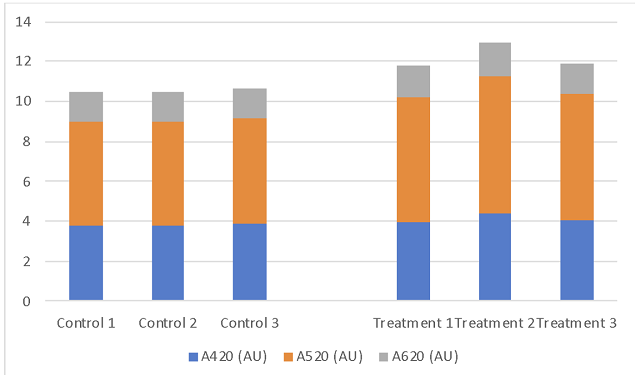
Table 5: Acid trials before fermentation and after aging
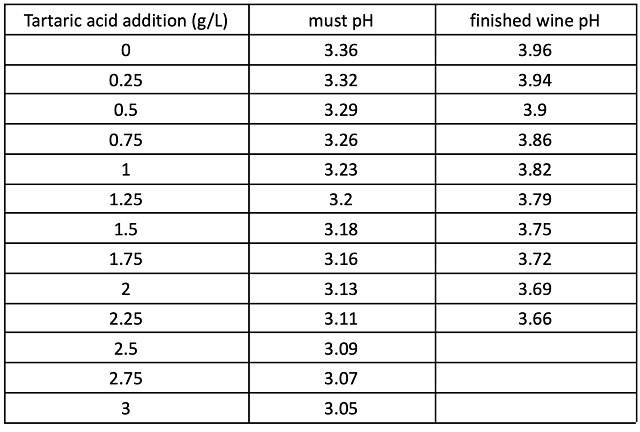
Table 6: Statistical analysis for descriptive scores from blind sensory analysis of Petit Verdot
References
(1) Wolf, T. K. Wine Grape Production Guide for Eastern North America; Plant and Life Sciences Publishing: Ithaca, New York, 2008.
(2) Robinson, J. The Oxford Companion to Wine, Third Edition.; Oxford University Press: Oxford, 2006.
(3) Carrie, D. Two Unsung Grapes Putting Virginia Wine on the Map. Wine Enthusiast. 2018.
(4) SMS Research Advisors. 2019 Virginia Commercial Grape Report. 2020.
(5) Ask the AWRI: Winemaking with High PH, High TA and High Potassium Fruit. Grapegrower and Winemaker 2018, October (657).
(6) Mpelasoka, B. S.; Schachtman, D. P.; Treeby, M. T.; Thomas, M. R. A Review of Potassium Nutrition in Grapevines with Special Emphasis on Berry Accumulation. Aust J Grape Wine Res 2003, 9 (3), 154–168.
(7) Gardner, D. Making (Red) Wine from Fruit High in Potassium. Penn State Extension Wine & Grapes U., 2016.
(8) Boulton, R. The General Relationship Between Potassium, Sodium, and PH in Grape Juice and Wine. American Journal of Enology and Viticulture 1980, 31 (2), 5.
(9) Gómez, J.; Lasanta, C.; Palacios-Santander, J. M.; Cubillana-Aguilera, L. M. Chemical Modeling for PH Prediction of Acidified Musts with Gypsum and Tartaric Acid in Warm Regions. Food Chem 2015, 168, 218–224.
