Exploring the effects of timing and amount of tartaric acid additions on chemical, microbial and sensory characteristics of Petit Verdot (Blenheim, 2021)
Kirsty Harmon
Blenheim Vineyards
Summary
Petit Verdot is the second most widely planted red grape variety in Virginia after Cabernet Franc, performing well in the vineyard and achieving ripeness and grape quality that lead to distinctive wines. However, even with tartaric acid additions, these wines sometimes complete fermentation with pH values higher than desired for longer aging wines. The most common way to bring wine pH into acceptable ranges in Virginia is tartaric acid addition, and common wisdom holds that earlier acid addition leads to lower risk of microbial spoilage as well as better integration of acidity. In this study, three approaches to acid adjustment in Petit Verdot acid were compared for chemical, sensory, and microbiological characteristics post aging. A control wine received no acid adjustment throughout the trial period. One treatment wine received 2 g/L of tartaric acid at crush while another had no acid adjustment at crush, with an acid adjustment post malolactic fermentation to the same target pH of the first treatment (1.25 g/L). Without acid addition, this wine completed fermentation with a pH of 3.78. An acid trial of the high pH wine showed that 1.25 g/L tartaric acid was needed to adjust the wine to pH of 3.56. After acid addition and aging, acidulated wines had comparable pH, potassium, tartaric acid, titratable acidity, and color while the unacidulated wine had higher pH, potassium and volatile acidity. In blind sensory analysis, the unacidulated wine received higher scores for fruit character, indicating more dark fruit while wine receiving acid addition at crush had brighter fruit character. There were no significant differences in sensory character with the timing of acid additions.
Introduction
Cabernet Franc and Petit Verdot are the two most widely planted red grape varieties in Virginia1. Both are bottled as a varietal wines and also used frequently in long aging Meritage blends. Despite having characteristics that make these good varieties to grow in Virginia vineyards2, both varieties have the potential to produce wines with high pH. In a survey of wines produced for WRE experiments from 2014 – 2020, the average pH for finished Petit Verdot wines was 3.85 and the average for Cabernet Franc was 3.76. Since these were finished, production scale wines, most of the wines included in this survey had been acidulated.
The likely cause of high pH in finished wines from these varieties is excess potassium in the grapes. Potassium in grapes is a function of soil, cultivar, vintage, rootstock, and farming techniques3. Though some interventions in the vineyard can reduce potassium, some variety/soil combinations will inevitably lead to high potassium juice with the potential to produce high pH wines.
When making red wine from fruit prone to high pH, the most commonly recommended approach is to add tartaric acid, however the magnitude and timing of additions can impact the resulting chemical and sensory characters of the wine. Both AWRI4 and Penn State Extension5 have publications advocating the addition of up to 4 g/L tartaric acid before fermentation in must that is known to have high potassium or comes from sites prone to high pH wines. Early addition allows for better retention of color and prevention of microbial spoilage common to high pH wines. However, lacking guidelines to determine how much acid to add, larger additions come with the risk of overly acidulation.
It is difficult to know at the beginning of fermentation how much acid is needed to achieve a final target pH. In a 2020 WRE study, Blenheim vineyards tested the effects of a large (4 g/L) tartaric acid addition to Petit Verdot from a vineyard known to produce high pH wines in the past. The acidulated wine completed malolactic fermentation with an average pH of 3.64 compared to 4.1 in the untreated wine. The acidulated wine had lower volatile acidity but also lower color. These wines were different in a triangle test. The acidulated wine had significantly higher scores for acidity and significantly lower scores for volume/body. Comments from winemakers indicated that the acidulated wine had been overly acidulated.
Several authors8,9 have developed equations for the prediction of final pH given various juice components, however these equations require measurement of juice components that are beyond the reach of most small to medium sized wineries and most predictions are only approximate. Gardiner5 recommends testing potassium to determine the amount of tartaric acid is needed, however no clear guidelines are given for tartaric additions based on potassium levels. The other approach is to add a smaller amount of tartaric acid initially, then more later. However, when pH<3.6, bitartrate precipitation has the effect of increasing wine pH, further exacerbating the issue.
Several WRE studies in 2021 examined the relationships among juice potassium, tartaric acid addition, wine pH and sensory characteristics. In this study, a single harvest lot of Petit Verdot was split into three treatments. The control received no acid addition at all. Treatment 1 receive no acid addition at crush, with acid adjustment post malolactic fermentation. Treatment 2 received 2 gl/L of tartaric acid at crush with no subsequent acid addition. Treatment 1 and Treatment 2 had the same target pH post adjustment.
Methods
Petit Verdot grapes from the Claim Vineyard (Blenheim) were hand harvested on 9/21 and chilled overnight then destemmed but not crushed into TBins with the addition of 50 mg/L SO2 (as a KMBS addition). Just prior to inoculation, juice samples were taken for general analysis (Brix, pH, TA) as well as an acid trial. To determine the malic acid, tartaric acid, and potassium of the juice, samples were shipped to ETS (St. Helena California). To prevent fermentation during transit, samples tubes were filled then weighed on a lab scale. To inactivate microbes, the tubes containing juice were briefly heated to boiling in the microwave, then weighed again. Any weight lost during boiling was replaced with distilled water to maintain the initial concentration of juice components.
Grapes were inoculated with 15 g/hL EC1118 yeast the day after processing (9/23). After two days of fermentation (9/25), 2 g/L tartaric acid was added to T2 bins only. Fermentations were monitored for °Brix and temperature each day following cap management. Bins were punched down twice daily throughout the fermentation until Brix measure lower than -1.5°. Once negative Brix had been reached, fermentations were pressed. Bins 1&2 were pressed together while bin 3 was pressed separately. Free run and press fractions were combined for each bin.
Wine was allowed to settle then transferred to comparable barrels and inoculated with 0.01 g/L Scott Labs MBR process. Malolactic fermentation was complete (malic acid tested 0.05 g/L or less by enzymatic analysis) on 10/17. An acid trial was done at this time to determine the amount of tartaric acid addition to be made to the control to obtain the same pH as the treated wine. SO2 (100 mg/L) was added four weeks after (on 11/18). Tartaric acid (1.25 g/L) was added to one barrel (T1) also on 11/18.
Sensory analysis was completed by a panel of 19 wine producers. Wines were presented blind in randomly numbered glasses. Tasters were presented with three different wines. There were four tasting groups to balance order effects. Tasters were asked to score each wine on a scale of 0 to 10 for fruit intensity, fruit character, acidity, astringency, and body/volume. They were also given open ended questions to describe the wines. Descriptive scores were analyzed using repeated measures ANOVA.
Results
Three TBins of Petit Verdot must were used for this experiment. Juice analysis showed a range from 23.2 – 24.4°Brix, a pH range of 3.48 – 3.55, and a potassium range of 1250-1340 mg/L (Table 1). Past juice analysis at crush and after cold soak has shown large differences in potassium and pH values due to the amount of extraction that has been allowed to occur (Lost Creek 2020). After juice analysis, 2 g/L tartaric acid was added to bin 3 (T2).
Post-fermentation wine chemistry (Table 2) shows that, despite receiving 2 g/L tartaric acid at crush, T2 wine only has 0.4 g/L more tartaric acid than the control. This addition also resulted in 0.2 unit decrease in pH and 250 mg/L decrease in potassium. There was no large difference in volatile acidity at this stage.
Acid trials were conducted post fermentation prior to malolactic fermentation as well as after malolactic fermentation (Table 3). For this experiment, further acid addition (to T1) was reserved until after malolactic fermentation due to the uncertainties in estimating final pH with malic acid conversion. Acid trials completed post-malolactic fermentation indicated an addition of 1.25 g/L tartaric acid was needed to achieve the same pH as T2 (Tables 3 & 4).
Acid chemistry in January shows that lower acid addition is needed post-fermentation (1.25 vs. 2.0 g/l) to achieve the same pH, TA and potassium levels compared to when acid is added at the crusher (Table 4). There was no decrease in volatile acidity with early addition shown in this experiment. One benefit of tartaric acid addition is a higher level of molecular SO2 throughout aging, also leading to better color retention (Table 5). There were only slight differences in color intensity in April (Figure 1), which may be due more to pH and free SO2 levels than differences in actual color retention.
At the end of the aging period (April), T1 and T2 had comparable pH, tartaric acid and titratable acidity (Table 6). Potassium was lower for both treatments that received tartaric acid additions. These differences did not notably change most microbial counts, though the control wine had 1690 cells/mL of Brettanomyces bruxellensis while the treatment barrels had 50 (T1) and 220 (T2) (Table 7). These levels are still well below the population density thought to cause spoilage.
Table 8 and Figure 2 depict changes in potassium, pH and volatile acidity during the various stages of wine production and aging. As expected, potassium and pH levels are the highest in the control throughout the time period measured. However, even without tartaric acid addition, potassium levels decline in the control during aging. After addition of tartaric acid to T1, potassium and pH levels are nearly the same as T2. Volatile acidity was not diminished by early intervention in pH values.
In theory, one potassium cation should bond with one bitartrate anion to form potassium bitartrate. By this logic, there should be an equal amount of potassium and tartaric acid lost from the wines. However, when measured values are compared, far more tartaric acid is lost than potassium (Table 9). Juice potassium is probably not a full measure of available potassium, as this cation continues to be added as more skin cells burst during fermentation. Therefore, though initial juice potassium may be helpful in determining if a wine is a candidate for early tartaric acid addition, it cannot be used as an exact measure of tartaric acid precipitation or estimation of final pH.
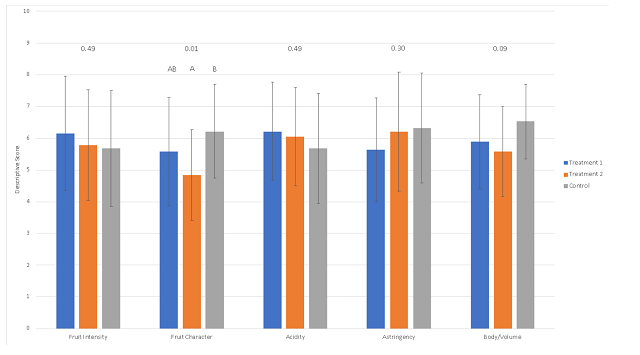
Sensory evaluation showed significantly higher scores for fruit character in the control compared to T2. T1 scored intermediate values for fruit character (Table 10, Figure 3). “Fruit character” was defined as “bright/fresh/red” (lower numbers) to “dark/dried/black”, indicating that higher pH wine presented with more dark fruit while wine receiving acid addition at crush had brighter fruit character. This effect appears to be independent of perceived acidity, as this measure was not significantly different among treatments.
Table 1: Juice chemistry for three bins of Petit Verdot (ETS)
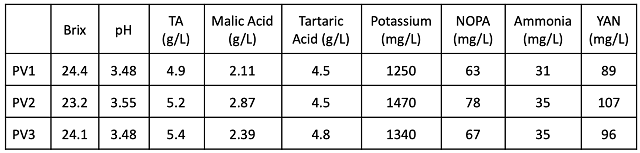
Table 2: Wine chemistry post-fermentation for two treatments of Petit Verdot
(ETS Labs, September 24)
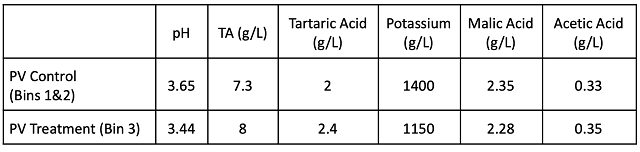
Table 3: Acid trials for treatment 2 (in-house data)
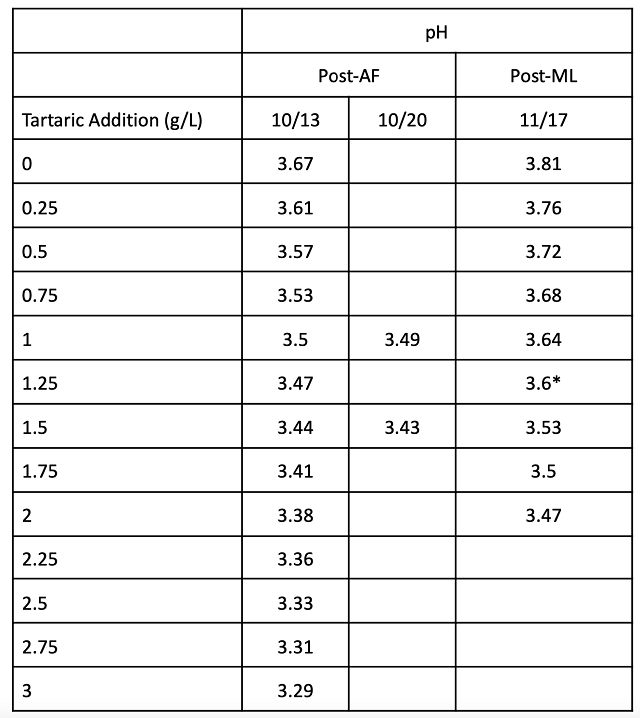
Table 4: Acid chemistry for three treatments of Petit Verdot (ICV labs Jan 2022)
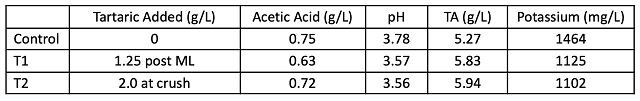
Table 5: Sulfur chemistry for three treatments of Petit Verdot (ICV Labs Jan 2022)
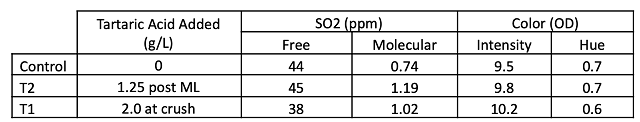
Figure 1: Color intensity for three treatments of Petit Verdot (ICV Labs, April 2022). Values for intensity, hue, and free SO2 (ppm) are listed on the endcap of each column.
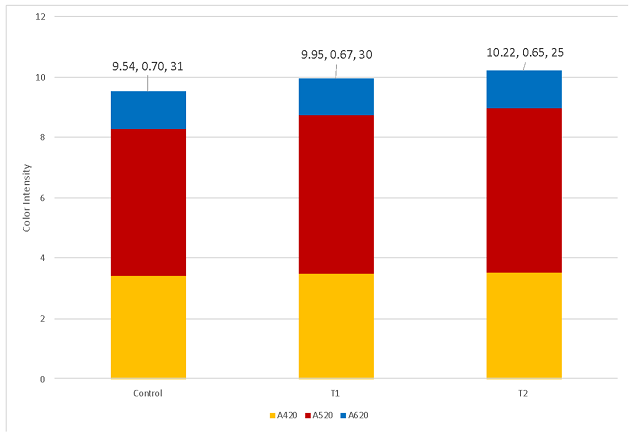
Table 6: Acid chemistry for three treatments of Petit Verdot (ICV Labs April 2022)
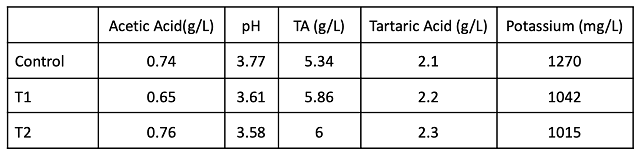
Table 7: Microbiology for three treatments of Petit Verdot (cells/mL) (ETS labs, April 2022)
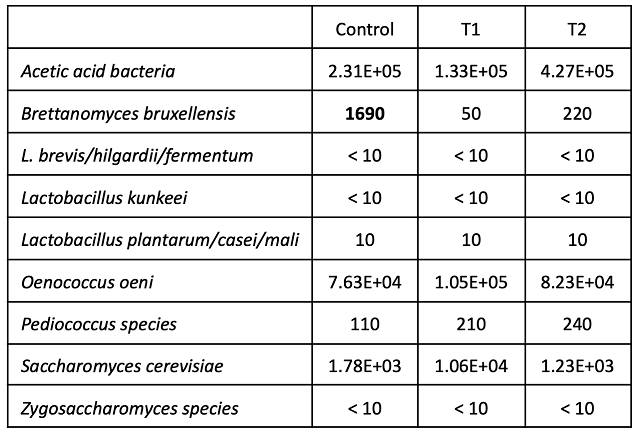
Table 8: Progression of acid parameters over time
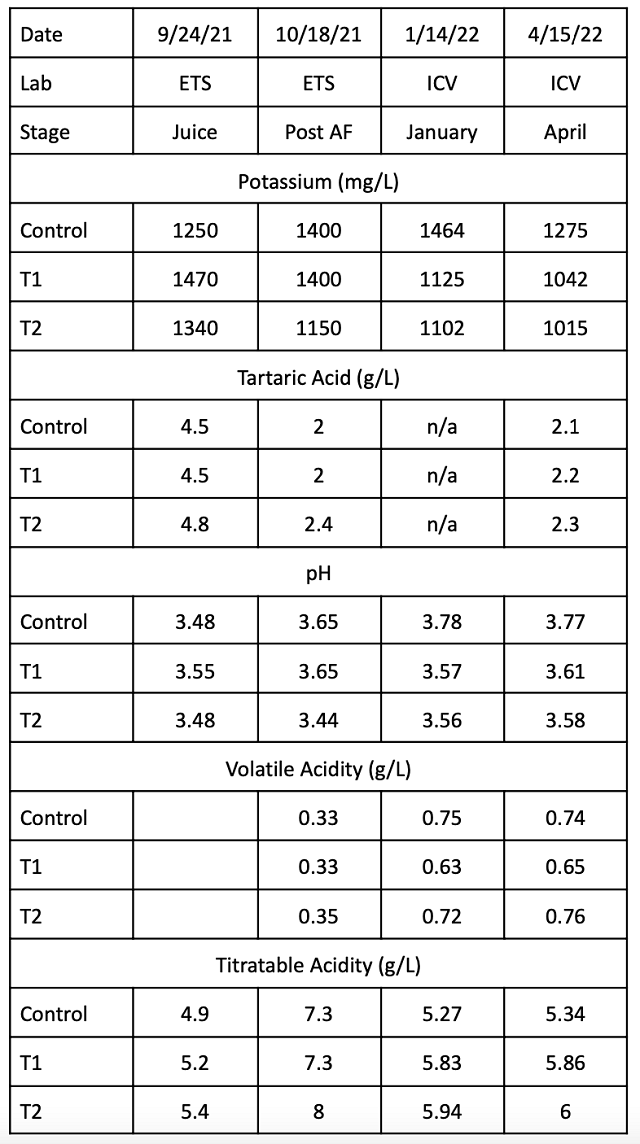
Figure 2: Progression of potassium, pH, and VA over time
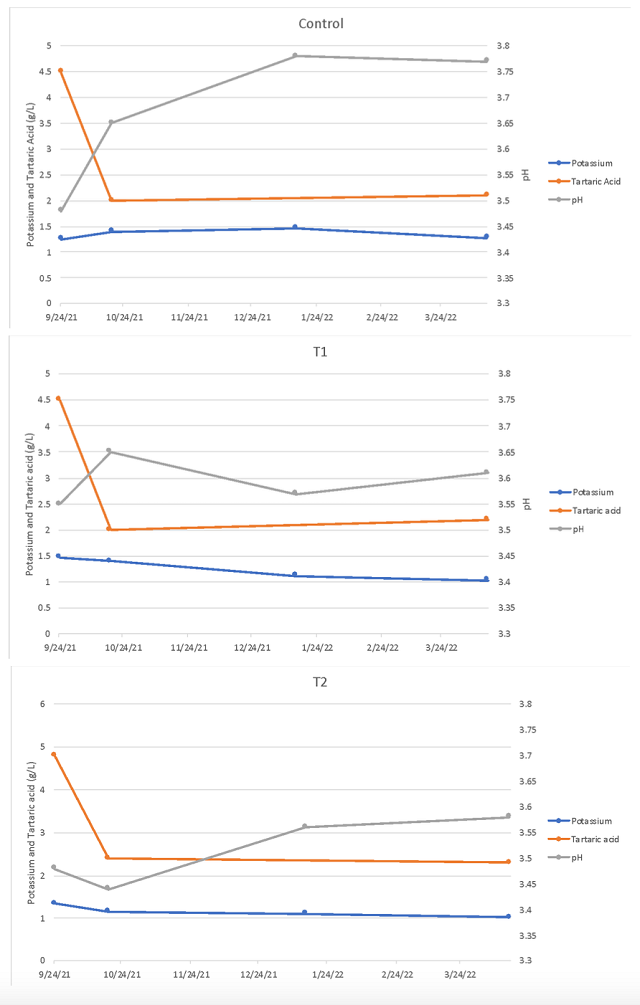
Table 9: Balance sheet for potassium and tartaric acid in two treatments of Petit Verdot (g/L)
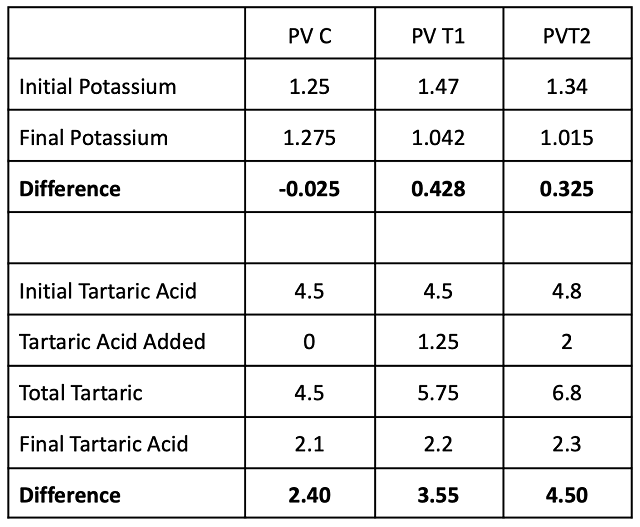
Table 10: Statistical analysis for descriptive scores from blind sensory analysis of Petit Verdot
Figure 3: Statistical analysis for descriptive scores from blind sensory analysis of Petit Verdot
References
(1) 2021 Commercial Wine Grape Report; Virginia Wine Board, Virginia Vineyards Association, Virginia Wineries Association, 2022.
(2) Wolf, T. K. Wine Grape Production Guide for Eastern North America; Plant and Life Sciences Publishing: Ithaca, New York, 2008.
(3) Moss, R. Potassium in Viticulture and Enology. Virginia Cooperative Extension Viticulture Notes 2016.
(4) Ask the AWRI: Winemaking with High PH, High TA and High Potassium Fruit. Grapegrower and Winemaker 2018, October (657).
(5) Gardner, D. Making (red) wine from fruit high in potassium. Penn State Extension Wine & Grapes U. https://psuwineandgrapes.wordpress.com/2016/09/23/making-red-wine-from-fruit-high-in-potassium/.
