Comparing Yeast Strains for Viognier: ICV GRE (Scottlabs) and X-16 (Laffort) (2018)
Emily Pelton
Veritas Vineyard and Winery
Summary
In 2011, the Virginia Wine Marketing Office began promoting Viognier as Virginia’s signature grape. ICV GRE (Scottlabs) yeast is popular for fermentation of Viognier due to its production of an aromatic profile that has become familiar in Virginia Viognier. However, winemakers report difficulty with stuck or sluggish fermentation in GRE inoculated Viognier fermentations. In this experiment, the aromatic profile of Viognier produced with ICV GRE was compared with that produced from the same juice using X-16 yeast (Laffort). Three barrels were inoculated for each yeast group. All three barrels inoculated with GRE yeast stuck with and average of 9.4 gh/L residual sugar while all three barrels inoculated with X-16 yeast finished completion (with residual sugar <0.4 g/L). The GRE barrels also had higher volatile acidity, likely as a result of yeast stress or longer time without SO2. In a sensory panel, there were no significant differences in scores for fruit intensity, floral intensity or overall aromatic intensity. There was also no significant difference in preference between the yeast groups. Potential reasons for stuck fermentation are discussed.
Introduction
Viognier is a grape variety native to the Rhone region in France1 that is widely planted in Virginia2. This variety is known for aromas of stone fruit with tropical notes of pineapple and orange blossoms. Though the aromatics suggest sweetness, the wine is fermented as a dry table wine. In 2011, varietal Viognier was bottled by 76 of the then 196 wineries in the state3. In May of that that year, the Virginia Wine Board approved marketing of Viognier as Virginia’s signature grape3. As a delicate, aromatic white wine, winemaking choices such as harvest date, fermentation temperature, skin contact and yeast strain can make a significant impact on the resulting wine. In this study, the same Viognier juice was fermented with two different commercially available yeast strains marketed for aromatic white wines: ICV GRE (Scottlabs) and X-16 (Laffort).
Saccharomyces cerevisciae, the primary species of yeast used in commercial wine production, likely originated from a single domestication event in Mesopotamia around the same time as domestication of wine grapes themselves4. This line of fermentative yeast spread around the world along with the grape vines it had evolved to transform into wine. Though there have been a few genetic bottlenecks leading to rapid, widespread adoption of a single genetic type, (such as the evolution of sulfite resistance in the Middle Ages) this species remains very diverse, such that no one strain accurately portrays the whole species4.
The primary metabolic function of Saccharomyces cerevisciae in wine production is the metabolism of sugar into ethanol with the coupled release of energy. However, complex cellular machinery is also at work to build and maintain cellular components through alternative pathways. It is the work of these pathways that leads to the production of volatile aromas and flavors. At any given time, hundreds of substrates are taken up into the yeast cell, transformed by enzymatic pathways, with end products expelled into the environment or used within the cell. It is differences in the functioning and regulation of these enzymes that produce the myriad of commercially available yeast strains. Though most S. cerevisciae strains share most pathways in common, the extent to which any one pathway functions is determined by both genetic differences and environmental influences.5 Different strains have different number of copies or slightly different coding for enzymes in side pathways, leading to large differences in nutrient demand, environmental tolerances, and the amount of any one end product. For example, some strains of S. cerevisciae metabolize malic acid as part of the malo-ethanolic pathway while others have been shown to product malic acid5.
It was Louis Pasteur who first identified S. cerevisciae as the primary fermentative yeast in wine in 1860. Since that time, many thousands of strains have been isolated, selectively bred and cultured for a wide range of characteristics and made available for commercial use. Some are selected for production of thiols (with high copy number of B-lyase genes) while others are tolerant to high levels of alcohol or acidity. Others boast the ability to help with color fixation or to restart stuck fermentations. Regardless of the source of the yeast strain, it is wise to heed this following advice from Ronald Jackson:
“The main characteristics of most commercial strains are know, but most of their other properties are not, or if known they are buried in research papers not readily accessible to most winemakers. The local conditions often are crucial to feature expressions. It is again up to the winemaker to do their own individual experimentation to determine what best suits their situations and preferences.”
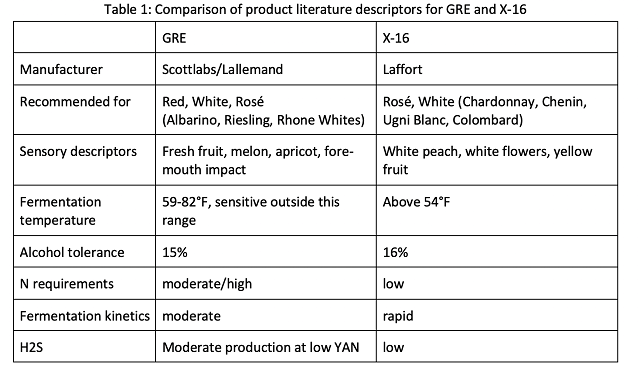
In this study, wines made from two commercially available yeast strains were compared: ICV GRE (Scottlabs) and Zymaflore X-16 (Laffort). GRE yeast is a strain of Saccharomyces cerevisiae var. cerevisiae that is frequently used in Virginia for Viognier fermentations. Selected from the Cornas area of the Rhone Valley, this yeast is recommended for white, rose, and red wines. Product literature states that in “fruit-focused whites, such as Chenin Blanc, Riesling and Rhone whites, ICV GRE fermentations result in stable, fresh fruit characteristics such as melon and apricot while improving fore-mouth impact.6” Product literature also specifically mentions this yeast for Riesling and Rousanne, however it is not one of the yeasts selected by Scottlabs for Viognier6.
GRE is currently the preferred Viognier yeast at Veritas Vineyards and Winery due to the aromatic profile of the wine it produces. However, the winemaker reports several instances when GRE has not completed fermentation, leaving higher than desired residual sugar (Emily Pelton, personal communication). Stuck and sluggish fermentation also sometimes leads to higher than desired levels of volatile acidity, leading the winery to explore an alternative yeast strain for Viognier.
X-16 (Laffort) is a strain of Saccharomyces recommended for production of modern white and Rosé wines. Product literature states it was bred for the “production of fermentative esters (white peach, yellow fruit) while retaining a sharp, clean aromatic profile” and promises “very high fermentative aroma production”, even from aromatically neutral grape varieties with high vine yield7. It is specifically recommended for Chardonnay, Chenin Blanc, Ugni Blanc, and Colombard7. Table 1 compares properties and recommendations for these two yeasts.
The purpose of this experiment was to compare fermentation kinetics and aromatic production when each yeast strain was used to ferment the same Viognier juice.
Methods
Grapes were harvested on August 29 and chilled overnight. On August 30, grapes were whole cluster pressed to a single tank with 70ppm SO2 added at the press pan. The hard pressings were separated from free run and lighter pressings at cycle 20; only free run and lighter pressings were used for this experiment. Cinn Free (1.6 ml/hL) and Stab Micro M (15 g/hL) were added to the tank. Juice was cold settled for two days before racking off lees to a separate tank. Juice was then racked to two separate barrel lots for fermentation. Juice chemistry was determined after cold settling. All racking steps were conducted with care to remain as anaerobic as possible, including use of inert gas.
All winemaking steps were kept the same for both lots with the exception of the yeast strain used for inoculation. Yeast (25 g/hL) was rehydrated in Fermo Plus Energy Glu (6 g/hL). Fermentation was monitored daily using density and temperature. Wine was analyzed at the winery lab on September 26, when fermentation kinetics ceased to show density depletion. This corresponded to completed fermentation (residual sugar <1.0 g/L) for X-16, however GRE had not completed fermentation at that time. Sensory analysis at this stage led to the decision to rack the wine (on October 4). Further attempts to finish the fermentation of the GRE barrels were stopped on October 24 with the addition of 40 ppm SO2 to all barrels. Three barrels per lot were analyzed for chemistry. A composite sample was used for sensory analysis.
Sensory analysis was completed by a panel of 31 wine producers. Wines were presented blind in randomly numbered glasses. Panelists were presented with two wines, one of each type, and asked to identify which wine they preferred (a triangle test). Due to differences in residual sugar between wines, sensory analysis was done for aromatic traits only. Participants were instructed to smell but not taste the wines. There were three tasting groups with the unique wine in the triangle test balanced between groups. Participants were then asked to score each wine on a scale of 0 to 10 for fruit intensity, floral intensity and overall aromatic intensity. They were also given open ended questions to describe the wines. Results for the triangle test were analyzed using a two-tailed Z test. Descriptive scores were analyzed using repeated measures ANOVA.
Results
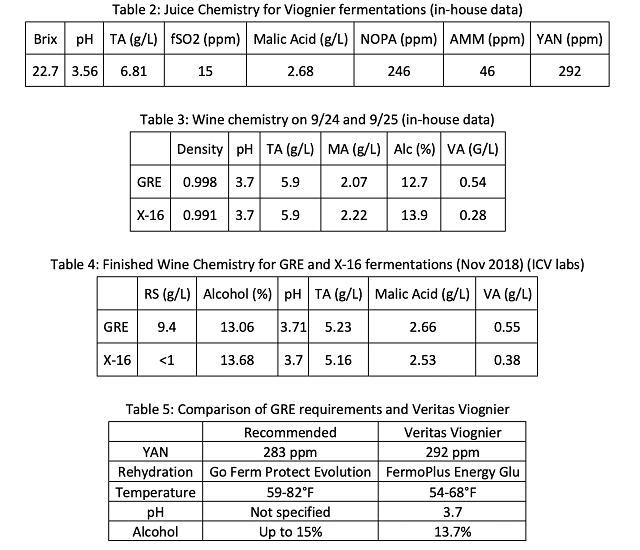
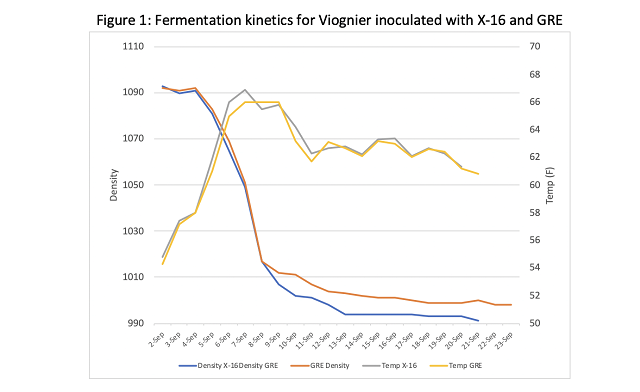
Juice for both treatment groups originated from the same settling tank. Juice chemistry is reported in Table 2. YAN was sufficient for fermentation according to the Scottlabs guidelines6, so no nutrient additions were made. Fermentation kinetics can be seen in Figure 1. Fermentations progressed along the same trajectory from September 2 through September 8, at which time the GRE fermentation slowed suddenly. After September 8, X-16 fermentation continued to progress to dryness while the GRE fermentation languished. Wine was analyzed at the winery lab on September 26, when fermentation kinetics ceased to show density depletion (Table 3).
Wine chemistry at the completion of density depletion can be seen in Table 3. Wine fermented with GRE had higher density than wine fermented with X-16. Three barrels from each lot were tested individually at that time to determine if all barrels showed the same level of completion. GRE barrels registered RS levels of 9.8, 9.9 and 10.1 g/L while all three X-16 barrels registered RS levels less than 0.1 g/L (ICV labs), indicating this is a consistent effect, not a single stuck barrel. A composite of the finished wine (after SO2 addition) was analyzed for each lot (Table 4). The difference in alcohol between the two lots is that which would be expected due to the differences in residual sugar, according to the formula8:
Potential Alcohol (%) = (glucose + fructose)(g/L)/16.83
Using this formula, a 0.56% difference in alcohol is predicted between the two wines. The difference is 0.62%. The GRE has higher volatile acidity, which may be due to yeast stress near the end of fermentation.
In a paired preference test of wines produced by two different yeast strains, 10 participants preferred the wine produced by X-16 yeast while 15 preferred the wine produced by GRE. These preferences were not significantly different (Z=0.8, p= 0.42). There no significant differences in scores between the wines for fruit intensity (F=0.01, p=0.93), floral intensity (F=2.8, p=0.14) or overall aromatic intensity (F=0.36, p=0.55).
Why did GRE Stick?
There are several reasons a fermentation may become sluggish or stick9. These include:
- Nitrogen limitation
- Ethanol limitation
- Extremes in temperature
- pH extremes
- Fructose buildup due to yeast glucose preference
- Toxicity factors
In the case of GRE with Viognier at Veritas, all of the stated conditions for fermentation were met. Stress is cumulative, and having multiple parameters close to the limit can also cause fermentation to languish, however none of these parameters was particularly close. Though the initial temperature was colder than the specified range, fermentation began quickly and proceeded at a healthy pace.
When a fermentation proceeds normally and stops abruptly, it is usually indicative of an abrupt shock, such as temperature extreme10, however that was not the case here. Nutrient limitation can also present as a slow end to fermentation, however, these grapes provided adequate nitrogen. Overall amount of nitrogen may not be the only consideration. Dr. Nichola Hall points out that “focusing on nitrogen alone is not the whole story, it correlates well with fermentation rate and biomass production but not fermentation security, especially in the later stages of fermentation” (personal communication). Rather, Dr. Hall encourages focusing on proper rehydration with nutrient that also provides balanced vitamins and minerals (like Scottlabs Go Ferm Protect Evolution). She also points out that low nutrient requiring yeast don’t perform well in high nitrogen must, and vice versa.
Conclusions
- X-16 (Laffort) completed fermentation with normal kinetics while the fermentation inoculated with GRE became sluggish and eventually stopped with 10 g/L of residual sugar remaining.
- The extended end to fermentation likely led to the higher levels of acetic acid in the GRE fermented wine.
- There was not significant difference in preference between the wines. Scores for fruit intensity, floral intensity and overall aromatic intensity were not significantly different.
References
(1) Viognier. Virginia Wine. https://www.virginiawine.org/varietals/viognier. Accessed 2/19/19
(2) Wood, V.; Custer, S.; Watson, K.; Alper, D. VIRGINIA 2018 COMMERCIAL GRAPE REPORT. 11.
(3) Wayne, N. The Best Virginia Viognier Wines. Washingtonian. November 22, 2011.
(4) Bisson, L. F. Geographic Origin and Diversity of Wine Strains of Saccharomyces. American Journal of Enology and Viticulture 2012, 63 (2), 165–176.
(5) Jackson, R. S. Wine Science: Principles and Applications, 4th edition.; Academic Press: Amsterdam, 2014.
(6) Scottlabs. Fermentation Handbook; 2018.
(7) Laffort. Xymaflore X-16. Laffort Product Information. https://lffort.com/en/product/zymaflore-x16/. Accessed 2/19/19
(8) ETS. Predicting Potential Alcohol. ETS. https://www.etslabs.com/library/8. Accessed 2/19/19
(9) Wann, G. Lecture 6A Stuck and Sluggish Fermentations. In Wine Production; 2014.
(10) Bisson, L. F.; Butzke, C. E. Diagnosis and Rectification of Stuck and Sluggish Fermentations. Am J Enol Vitic. 2000, 51 (2), 168–177.
