Comparing chemical and sensory characteristics of Cabernet Franc wines fermented with and without addition of commercial yeast (2020)
Todd Henkle
The Vineyard and Winery at Lost Creek
Summary
At the Vineyards and Winery at Lost Creek, red wines have been fermented without the addition of commercial yeast since 2016. They believe that the greater diversity in the microbial population during ambient fermentation leads to more complex finished wines. However, ambient fermentation also includes a greater level of risk due to microbial spoilage, and can lead to elevated levels of acetic acid and ethyl acetate. The purpose of this experiment was to compare wines fermented with two different commercial yeast strains with wine fermented without commercial yeast inoculation. Wines produced by commercial yeast had lower levels of acetic acid and ethyl acetate than uninoculated wines. These differences were evident as early as the completion of alcoholic fermentation. In a triangle test of inoculated vs. uninoculated wine, the wines were significantly different, however there were no significant differences in scores for aromatic intensity, fruit intensity, volume/body, complexity, or perception of volatile acidity.
Introduction
At the Vineyards and Winery at Lost Creek, red wines have been fermented without the addition of commercial yeast since 2016. They believe that the greater diversity in the microbial population during ambient fermentation leads to more complex finished wines. However, ambient fermentation also includes a greater level of risk due to microbial spoilage, and can lead to elevated levels of acetic acid and ethyl acetate.
Grapes enter the winery covered in microbes from the vineyard. To date, 52 different species of yeast from 22 different genera have been identified on grapes including Hanseniospora (AKA Klockera), Candida, Pichia, Hansenula, Metschnikowia, Sporoblomyses, Cryptococcus, Thodotorula, and Aurobasidium1. The cast of characters changes as grapes ripen, with the greatest abundance of microbes present in the last few weeks on the vine1. The overall inoculant of non-Saccharomyces yeast and bacteria coming into the winery from the vineyard on the grapes is often larger than the inoculant of selected Saccharomyces yeast added at the beginning of fermentation with a commercial yeast1.
These microbial hitchhikers have several impacts on the wine, both positive and negative. Klockera apiculata (aka Hanseniaspora uvarum) is a common member of the non-Saccharomyces yeast community found on grapes1,4. This yeast strain is tolerant to up to 100 mg/L SO2, can grow at low temperature (such as that found during cold soak), and can produce both acetic acid and ethyl acetate (which smells like nail polish remover) under aerobic conditions3. Other offenders in the non-Saccharomyces yeast community include Pichia guilliemondii, a film forming yeast prevalent in warm conditions when fermentation is delayed. This yeast can form spores that become resident in barrels and produce ethyl acetate and 4 ethyl phenol (which can smell like band-aid, wet dog, horse sweat)1–3. In addition to yeast, many spoilage bacteria that can produce acetic acid, mousy flavor and biogenic amines (which have names like putrescine and cadaverine…) also come into the winery on grapes.
Despite the risks, there are also some benefits to having a rich microbial community early in fermentation. Several non-Saccharomyces yeast species have been shown to produce positive compounds that add complexity to wine aroma such as esters, higher alcohols, glycerol, succinic acid and thiols. Proteases produced by non-Saccharomyces yeast have been shown to break down cells and add nutrients, ultimately making a more protein stable wine. Some produce glycosidases that help unmask aromas compounds that are bound to sugar molecules. Others produce enzymes to break down polysaccharides that would otherwise inhibit clarification and filtration. Lachanacea thermotolerans has been shown to consume acetic acid, reducing volatile acidity1,5,6. It is likely these are some of the mechanisms that occasionally lead winemakers to employ ambient fermentations.
Many of our winemaking decisions affect the abundance and diversity of the microbial community present at the beginning of fermentation. As soon as the grapes are crushed, nutrients are released to feed the organisms that are present. The low pH environment of the juice, rising alcohol, rising temperatures, and presence of phenolics also tends to inhibit spoilage organisms in early fermentation. Uninoculated fermentations usually include prolonged time before Saccharomyces takes over, allowing plenty of time for other microbes to be active. Inoculation with commercial yeast hastens the exclusion of oxygen needed to form acetic acid and ethyl acetate, as well as the production of alcohol that can lead to inhibition of spoilage microbes. However, that also limits the time for a rich microbial population to produce complex aromas. The purpose of this experiment was to compare wines fermented with two different commercial yeast strains with wine fermented without commercial yeast inoculation. A fourth wine was fermented with a sequential inoculation of Torulaspora delbrueckii (BioDiva, Scottlabs) followed by commercial Saccharomyces (ICV D254).
Methods
Fruit was hand harvested and grapes were refrigerated for 2 days prior to processing. Fruit was sorted then destemmed into three Tbins with 10% whole cluster inclusion, 45% whole berry and 45% crushed fruit. SO2 (40ppm) and Stab Micro M (2 g/hL) were added at this time. A juice sample was collected from each TBin for general chemistry. A 10% saignee was done on each bin, with removal of 51 liters of juice. Bins were covered in plastic wrap and gassed, then returned to the refrigerator for two days of cold soak. When bins were brought out of the refrigerator, a second set of juice samples was taken for general chemistry and microbiology.
Samples were sent to ETS labs in California for microbiological analysis. Freezing kills off some microbes, and although some will survive, they do not generally represent the entire population that was present before freezing. Rich De Scenzo from ETS recommended a method whereby samples were centrifuged, the juice was poured into a new tube, leaving a small amount on the pellet, capping both tubes and shipping them to ETS overnight, thus preventing fermentation during shipment. The samples were reconstituted upon arrival at ETS.
After cold soak:
-
One bin was left in the cellar without inoculation to ferment as an ambient fermentation.
-
One bin was inoculated with 20 g/hL Uvaferm BDX yeast (Lallemand) rehydrated in 20 g/hL Superstart Rouge (Laffort).
-
One bin as inoculated with 20 g/hL ICV D254 (Lalvin) yeast rehydrated in 20 g/hL Superstart Rouge (Laffort).
-
A fourth lot was included as a demonstration wine only. In this lot, wine was initially inoculated with Torulaspora delbrueckii (Biodiva, Scottlabs). Three days later, after 3.5° Brix depletion, the TBin was inoculated with ICV D254 yeast (Lalvin). Other differences in protocol include a foot stomping after cold soak and addition of medium toast oak staves to barrels during malolactic fermentation.
Acid additions were made to a common target pH. The two inoculated bins received acid additions on the day after inoculation (3.0 g/L for BDX and 2.8 g/L for D254). The non-inoculated bin received its acid addition 4 days later, when signs of fermentation began. Bins were lightly punched down and gassed until signs of fermentation began, after which they received two punchdowns daily. Bins underwent extended maceration with 30-33 days of skin contact. Wine was gassed daily after the completion of alcoholic fermentation. After pressing, wine was allowed to settle, then transferred to comparable oak barrels for malolactic fermentation. Malolactic fermentation was determined complete on the non-inoculated lot in December. The two inoculated lots were not finished, so VP41 malolactic bacteria were added to speed completion. These completed malolactic fermentation in March. SO2 (70 ppm) was added to the barrels at the time malic acid depletion was determined to be complete.
Sensory analysis was completed by a panel of 17 wine producers. Due to restrictions put in place during COVID-19, sensory analysis was completed using shipped samples. Each wine producer received three wines in identical bottles, filled on the same day, each coded with random numbers. Two of the bottles contained the same wine while the third bottle contained the different wine. Participants were asked to identify which wine was different (a triangle test). There were four tasting groups with the unique wine in the triangle test balanced among the groups. Participants were then asked to score each wine on a scale of 0 to 10 for aromatic intensity, fruit intensity, volume/body, complexity, and perception of volatile acidity. They were also given open ended questions to describe the wines. Results for the triangle test were analyzed using a one-tailed Z test. Descriptive scores were analyzed using repeated measures ANOVA.
Results
The chemistry of fruit samples was determined by in-house testing before and after cold soak (Table 1). In each case, Brix changed little during cold soak, while pH and potassium increased notably and titratable acidity dropped. YAN also appeared to increase during cold soak. Potassium and nitrogenous compounds are resident in skin cells, so it is expected these will be released over time. The chemistry of each of the bins was very similar, especially after cold soak.
Microbiology of the bins was also very similar after cold soak, before the beginning of fermentation (Table 1). It is notable that the prevalence of Saccharomyces cerevisciae is 100 times less than that of Acetic Acid bacteria at this time (Table 2). Saccharomyces is uncommon in vineyard settings, and it is possible even this smaller amount is coming from resident winery yeast.
Fermentation began shortly after inoculation with commercial yeast, with noticeable Brix depletion within three days (these were inoculated on 10/13) (Figure 1). The non-inoculated bin took longer to start fermentation, with the first sign of noticeable Brix depletion on 10/19. Once the fermentation began, however, it proceeded at a pace and accumulated heat similar to those of the inoculated fermentations.
Despite fermenting at a similar temperature, it appears that the non-inoculated bin had better alcohol conversion than the inoculated bins (13.4% alcohol vs. 13.1%, Table 3). These all finished with similar pH and TA values. Volatile acidity, however, was higher in the non-inoculated bin. When volatile acidity measurements are compared at each stage of winemaking (Table 4), it appears that the difference in volatile acidity in the finished wine first appeared during alcoholic fermentation. All of the lots have similar rate of VA accumulation during malolactic fermentation and aging. Volatile acidity accumulation during alcoholic fermentation may be due to the presence of spoilage yeast at work during the extra days prior to the start of fermentation, or it may be produced by the yeast themselves, especially if they were stressed as alcohol concentration increased and nutrients decreased.
Ethyl acetate is a spoilage compound with sensory properties of nail polish remover, glue or varnish. It is formed by a chemical reaction between ethanol and acetic acid that is catalyzed by Pichia, Hanseniaspora, as well as some lactic acid bacteria and acetic acid bacteria. The sensory threshold of detection is from 90-150 mg/L. Levels below this are sometimes perceived as sweet or lending complexity while levels above are considered a fault. Here, ethyl acetate levels of wines fermented with commercial yeast are below the threshold of detection for ethyl acetate while the non-inoculated lot is within the threshold range (Table 4).
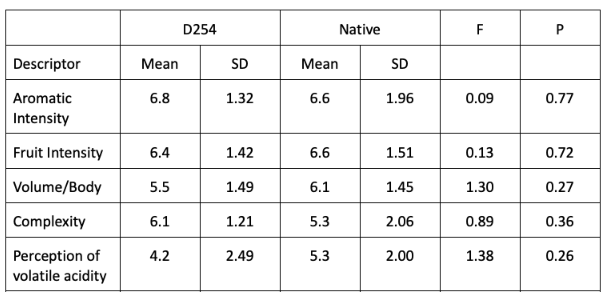
In a triangle test, 10 out of 17 respondents were able to distinguish which wine was different, indicating the wines were significantly different (Z=1.97, p= 0.024). There were no significant differences in scores for aromatic intensity, fruit intensity, volume/body, complexity, or perception of volatile acidity (Table 5).
Table 1: Fruit chemistry before and after cold soak for four yeast additions to Cabernet Franc (in-house data)
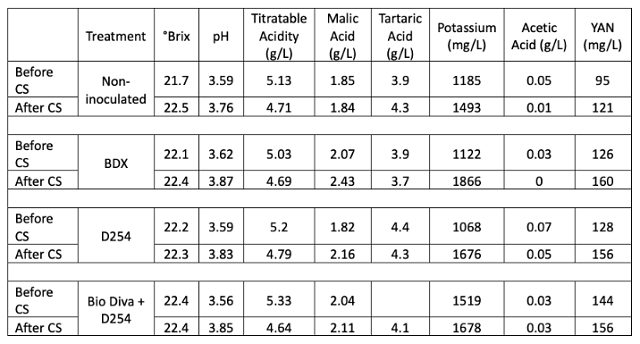
Table 2: Microbiology of fruit for three yeast treatments of Cabernet Franc taken 2 days after processing (ETS Labs)
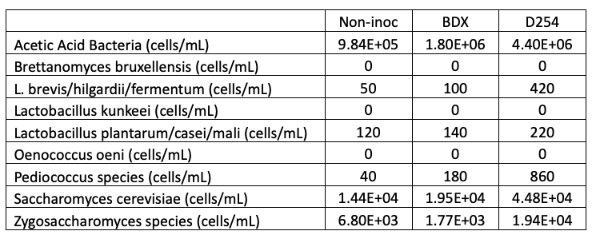
Figure 1: Fermentation kinetics for three yeast treatments of Cabernet Franc (in-house data)
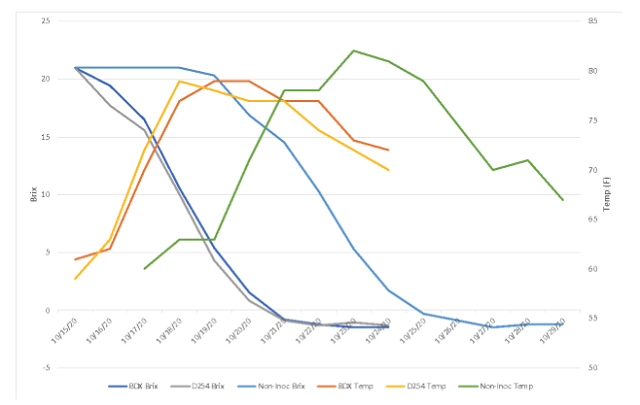
Table 3: Finished wine chemistry for three yeast treatments of Cabernet Franc (ICV Labs)
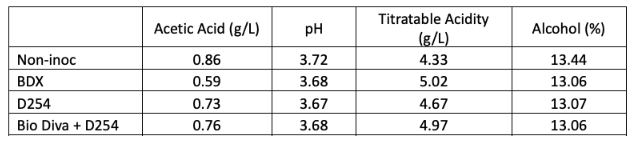
Table 4: Evolution of acetic acid (g/L) for three yeast treatments of Cabernet Franc. Ethyl acetate is reported in mg/L (in-house data and ICV labs)
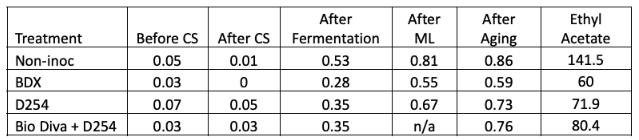
Table 5: Statistical analysis for descriptive scores from blind sensory analysis of Cabernet Franc
References
(1) Jackson, R. S. Wine Science: Principles and Applications, 4 edition.; Academic Press: Amsterdam, 2014.
(2) Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The Microbial Ecology of Wine Grape Berries. International Journal of Food Microbiology 2012, 153 (3), 243–259.
(3) Wine Microbiology. UC Davis Viticulture and Enology.
(4) Diversity of Wine Yeasts | Viticulture and Enology. https://wineserver.ucdavis.edu/industry-info/enology/wine-microbiology/yeast-mold/diversity-wine-yeasts (accessed 2018-11-13).
(5) Jolly, N. P.; Varela, C.; Pretorius, I. S. Not Your Ordinary Yeast: Non- Saccharomyces Yeasts in Wine Production Uncovered. FEMS Yeast Res 2014, 14 (2), 215–237.
(6) Vilela, A. Lachancea Thermotolerans, the Non-Saccharomyces Yeast That Reduces the Volatile Acidity of Wines. Fermentation 2018, 4 (3), 56.
