Comparing chemical and sensory characteristics in Cabernet Franc inoculated with non-Saccharomyces yeast (Biodiva), Saccharomyces yeast (BDX), and non-inoculated fermentation (2021)
Todd Henkle
The Vineyard and Winery at Lost Creek
Summary
At the Vineyards and Winery at Lost Creek, red wines have been fermented without the addition of commercial yeast since 2016. They believe that greater diversity in the microbial population during ambient fermentation leads to more complex finished wines. However, ambient fermentation also includes a greater level of risk due to microbial spoilage including elevated levels of acetic acid and ethyl acetate. In this experiment, wines produced from the same fruit were produced using (1) single inoculation with commercial yeast (BDX), (2) sequential inoculation with non-Saccharomyces yeast (Biodiva, Scottlabs) followed by commercial Saccharomyces yeast (BDX), and (3) no inoculation with commercial yeast. Inoculation led to faster onset of fermentation and lower concentration of acetic acid and ethyl acetate in the finished wine. Sensory analysis of all three wines showed no significant differences in sensory scores for any measures, including perception of volatile acidity and complexity.
Introduction
At the Vineyards and Winery at Lost Creek, red wines have been fermented without the addition of commercial yeast since 2016. They believe that greater diversity in the microbial population during ambient fermentation leads to more complex finished wines. However, ambient fermentation also includes a greater level of risk due to microbial spoilage including elevated levels of acetic acid and ethyl acetate.
Grapes enter the winery covered in microbes from the vineyard. To date, 52 different species of yeast from 22 different genera have been identified on grapes including Hanseniospora (AKA Klockera), Candida, Pichia, Hansenula, Metschnikowia, Sporoblomyses, Cryptococcus, Thodotorula, and Aurobasidium1. The cast of characters changes as grapes ripen, with the greatest abundance of microbes present in the last few weeks on the vine1. The overall inoculant of non-Saccharomyces yeast and bacteria coming into the winery from the vineyard on the grapes is often larger than the inoculant of selected Saccharomyces yeast added at the beginning of fermentation with a commercial yeast1.
These microbial hitchhikers have several impacts on the wine, both positive and negative. Klockera apiculata (aka Hanseniaspora uvarum) is a common member of the non-Saccharomyces yeast community found on grapes1,2. This yeast strain is tolerant to up to 100 mg/L SO2, can grow at low temperature (such as that found during cold soak), and can produce both acetic acid and ethyl acetate (which smells like nail polish remover) under aerobic conditions3. Other offenders in the non-Saccharomyces yeast community include Pichia guilliemondii, a film forming yeast prevalent in warm conditions when fermentation is delayed. This yeast can form spores that become resident in barrels and produce ethyl acetate and 4 ethyl phenol (which can smell like band-aid, wet dog, horse sweat)1,3,4. In addition to yeast, many spoilage bacteria that can produce acetic acid, mousy flavor and biogenic amines (which have names like putrescine and cadaverine…) also come into the winery on grapes.
Despite the risks, there are also some benefits to having a rich microbial community early in fermentation. Several non-Saccharomyces yeast species have been shown to produce positive compounds that add complexity to wine aroma such as esters, higher alcohols, glycerol, succinic acid and thiols. Proteases produced by non-Saccharomyces yeast have been shown to break down cells and add nutrients, ultimately making a more protein stable wine. Some produce glycosidases that help unmask aroma compounds that are bound to sugar molecules. Others produce enzymes to break down polysaccharides that would otherwise inhibit clarification and filtration. Lachanacea thermotolerans has been shown to consume acetic acid, reducing volatile acidity1,5,6. It is likely these are some of the mechanisms that occasionally lead winemakers to employ ambient fermentations.
Many of our winemaking decisions affect the abundance and diversity of the microbial community present at the beginning of fermentation. As soon as the grapes are crushed, nutrients are released to feed the organisms that are present. The low pH environment of the juice, rising alcohol, rising temperatures, and presence of phenolics also tends to inhibit spoilage organisms in early fermentation. Uninoculated fermentations usually include prolonged time before Saccharomyces takes over, allowing plenty of time for other microbes to be active. Inoculation with commercial yeast hastens the exclusion of oxygen needed to form acetic acid and ethyl acetate, as well as the production of alcohol that can lead to inhibition of spoilage microbes. However, that also limits the time for a rich microbial population to produce complex aromas.
At Lost Creek, the goal is to produce high quality, complex red wines, without spoilage. In 2020, an experiment comparing different fermentation strategies showed that fermentation took 3 days longer to start consuming a measurable amount of sugar without inoculation with commercial yeast. This wine completed fermentation with notably higher acetic acid. After malolactic fermentation and aging, the non-inoculated wine had 0.86 g/L acetic acid while three other wines from the same fruit, inoculated with different commercial yeast, had 0.59 – 0.73 g/L acetic acid. The ambient fermented wine also had notably higher ethyl acetate (141 mg/L) compared to inoculated wine (60 - 72 mg/L). However, these differences did not lead to any significant differences in perception of volatile acidity or complexity.
In the 2020 experiment, a demonstration wine was included that utilized a sequential inoculation with Biodiva (Scottlabs), a selected strain of Torulaspora delbrueckii. This non-Saccharomyces yeast strain is recommended by the manufacturer to enhance aroma and complexity of wine without the same spoilage risks as ambient fermentation. Torulaspora does not complete fermentation on its own, so it is used to begin fermentation with a Saccharomyces species inoculated after 2-3°Brix depletion. In 2020, there were other differences between the control and Biodiva treated wine that may have obscured the results, therefore it was only included as a demonstration. In 2021, Biodiva was included as part of the controlled experiment.
Methods
Cabernet Franc was harvested from 5 Nickels Vineyard in Bluemont, VA on 9/20. Fruit was chilled overnight before processing into TBins. Bins received 0.838 – 0.883 tons of fruit for an estimated 627 liters of wine (650 tons/Liter). Fruit included 50% whole berries and 50% crushed berries. At crush, all bins received 40 ppm SO2. After processing, 12% of the liquid (64-71 L, depending on overall weight of fruit added to the bin) was bled off, then bins were gassed thoroughly, sealed with plastic wrap and moved to the cellar to allow them to warm.
On 9/22, juice was taken for analysis. To sample for juice microbiology, a representative juice sample was taken after homogenization (punchdown) but before inoculation. The juice was centrifuged to form a pellet. Supernatant juice was poured into a new tube, leaving a small amount of juice on the pellet. Both tubes were capped and shipped overnight to ETS without freezing (which would kill the cells). Upon arrival, ETS reconstituted the sample before testing to maintain proper concentrations.
The same day (9/22), one bin was inoculated with 10 g/hL BDX yeast rehydrated in 10 ghL GoFerm Protect Evolution. A second bin was inoculated with 25 g/hL BioDiva. On 9/27, the Biodiva inoculated bin received 10 g/hL BDX yeast rehydrated in 10 g/hL GoFerm Protect Evolution. Bins were lightly punched down and gassed until signs of fermentation began, after which they received three punchdowns daily. On 9/24, 1.5 g/L tartaric acid was added to each bin. Fermentations were checked for Brix and temperature once daily. At the completion of fermentation, bins were gassed each day before pressing on 10/12 for a total of 21 days of maceration. After pressing, wine was allowed to settle, then transferred to comparable oak barrels for malolactic fermentation. Wine was treated with 70 ppm SO2 at the completion of malolactic fermentation. Native and Biodiva/BDX treatments completed malolactic fermentation on 12/3 while the BDX treatment completed fermentation on 1/5.
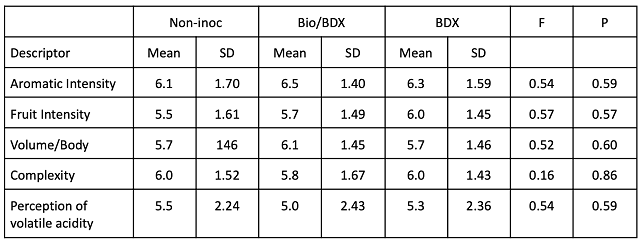
Sensory analysis was completed by a panel of 20 wine producers. Wines were presented blind in randomly numbered glasses. Tasters were presented with three wines, two of one type and one of another, and asked to identify which wine was different (a triangle test). There were four tasting groups with the unique wine in the triangle test balanced between groups. Tasters were then asked to score each wine on a scale of 0 to 10 for aromatic intensity, fruit intensity, volume/body, complexity, and perception of acidity. They were also given open ended questions to describe the wines.
Results
The initial chemistry for fruit in each of the treatments was nearly identical (Table 1). The fruit that was inoculated with BDX began fermentation sooner and completed alcoholic fermentation earlier than either of the other two treatments (Figure 1). The non-inoculated treatment did not show Brix depletion for six days. Once it began fermentation, it progressed at a pace comparable to the other two treatments. The bin that was initially treated with Biodiva began fermentation more slowly. The pace increased on 9/28, the day after BDX was added. Sequential inoculation is part of the use of non-Saccharomyces yeast, as this species of yeast is not meant to be fully fermentative. As a result of differences in fermentation kinetics, the fruit inoculated with BDX and Biodiva & BDX had many more days of post-fermentation (alcoholic) maceration than the fruit allowed to ferment without inoculation with commercial yeast.
After completion of fermentation, wine produced from inoculated fermentations (BDX, Biodiva & BDX) had lower acetic acid concentration than the non-inoculated wine (Table 2). The fermentation inoculated with BDX only had lower acetic acid than the Biodiva/BDX wine. This may be due to overall lower pH, or to stressed conditions at the end of Biodiva fermentation prior to BDX inoculation. After aging for several months, the wines produced by fermentation without commercial yeast had the highest level of acetic acid, while the wine produced by Biodiva & BDX inoculation had the lowest acetic acid concentration (Figure 2). Ethyl acetate is another component of volatile acidity, and is a common spoilage compound produced early in fermentation. The non-inoculated fermentation produced 147 mg/L ethyl acetate while the BDX fermentation produced 58 mg/L and the Biodiva/BDX fermentation produced 53 mg/L (ICV Labs, May 2022). According to ETS, the limit of detection of ethyl acetate ranges from 90-150 mg/L depending on the wine matrix (ETS labs), so these differences may or may not have been detectable.
Microbiological analysis of juice and aged wine shows that the fruit inoculated with Biodiva had an overall higher starting population of Hanseniaspora while the BDX fruit had higher values for Pichia species. Both are wild yeast that are often present in high levels on grapes. Hanseniaspora (aka Klockera) is lemon shaped and can be easily identified with a microscope. It can initiate fermentation and produces high levels of acetic acid and ethyl acetate as well as quickly use up nutrients. These yeast decline as fermentation begins due to lack of oxygen and rising levels of alcohol. Pichia is also fermentative, and can also produce acetic acid and ethyl acetate. In addition to coming in on grapes, it can also form films in barrels and tanks during storage.
Post-fermentation and aging, all of the barrels had a high level of other Lactobacillus plantarum/casei/mali (Table 3). These species do not usually produce acetic acid, but can form diacetyl, mouse taint and/or biogenic amines. Both barrels of the wine inoculated with BDX also had a notably higher load of Lactobacillus brevis/hilgardii/fermentum and Pediococcus species. The fact that these values are higher in both barrels of the treatment indicates the microbial population was higher after fermentation, rather than being caused simply by barrel contamination. L. brevis/hilgardii/fermentum can ferment glucose to lactic acid, acetic acid and CO2 and has the ability to produce high levels of acetic acid in a short period of time. Infections can occur readily during sluggish or stuck fermentations. Pediococcus are most often found in wines with high pH (>3.7). Some species of Pediococcus may produce polysaccharides that cause undesirable texture defects. Lactobacillus sp. can produce mousey flavors while both types of lactic acid bacteria can produce biogenic amines.
Despite the differences in chemistry and microbiology among the wines, there were no significant differences in sensory scores for aromatic intensity, fruit intensity, volume/body, complexity or perception of volatile acidity (Table 4), indicating these differences were below threshold or otherwise masked by other compounds in the wine. None of the fermentation conditions seemed to affect perception of complexity or spoilage.
Table 1: Juice chemistry for three treatments of Cabernet Franc (In-house data)
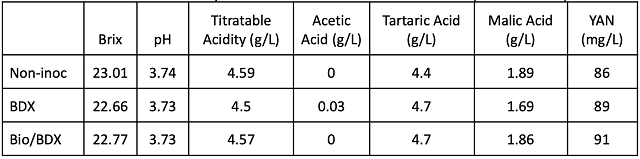
Figure 1: Fermentation kinetics for three treatments of Cabernet Franc (In-house data)
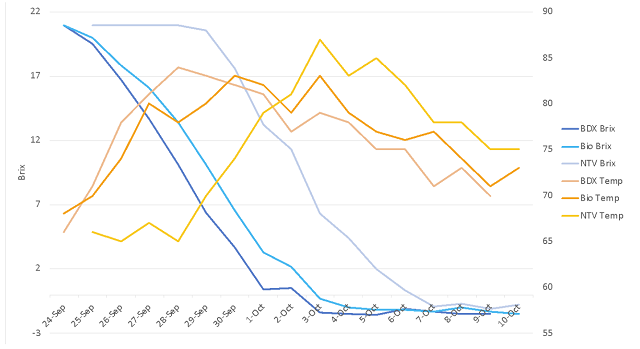
Table 2: Post-malolactic fermentation chemistry for three treatments of Cabernet Franc
(ICV Labs, Feb 2022)
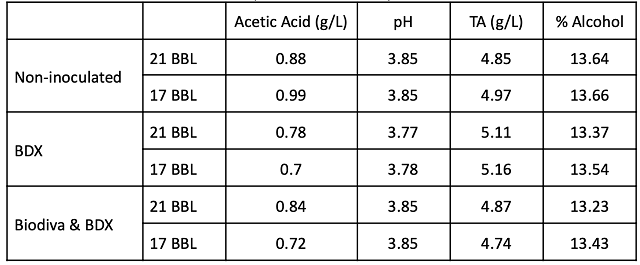
Figure 2: Comparison of acetic acid for four time points. Dec and Jan values were from in-house analysis. Feb and April values are from ICV labs.
Table 3: Comparison of microbes in juice and wine for three treatments of Cabernet Franc (ETS Labs). All values are reported as cells/mL.
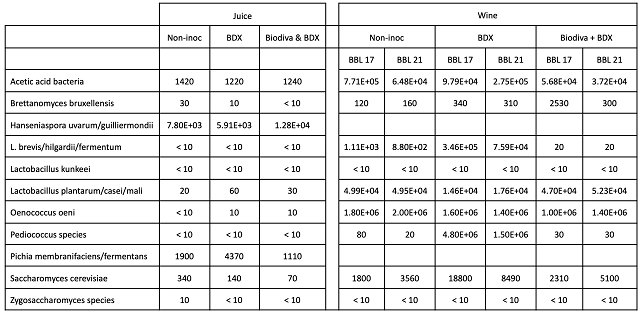
Table 4: Statistical analysis for descriptive scores from blind sensory analysis of Cabernet Franc
References
(1) Jackson, R. S. Wine Science: Principles and Applications, 4 edition.; Academic Press: Amsterdam, 2014.
(2) Diversity of Wine Yeasts | Viticulture and Enology. https://wineserver.ucdavis.edu/industry-info/enology/wine-microbiology/yeast-mold/diversity-wine-yeasts (accessed 2018-11-13).
(3) Wine Microbiology. UC Davis Viticulture and Enology.
(4) Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The Microbial Ecology of Wine Grape Berries. International Journal of Food Microbiology 2012, 153 (3), 243–259.
(5) Jolly, N. P.; Varela, C.; Pretorius, I. S. Not Your Ordinary Yeast: Non- Saccharomyces Yeasts in Wine Production Uncovered. FEMS Yeast Res 2014, 14 (2), 215–237.
(6) Vilela, A. Lachancea Thermotolerans, the Non-Saccharomyces Yeast That Reduces the Volatile Acidity of Wines. Fermentation 2018, 4 (3), 56.
