Protein folding explains protein instability in wine
Joy Ting
October 2019
Protein instability in wine is the appearance of an “amorphous haze or deposit” in the wine (1). Chemically, the haze is made up of complexes of proteins, polysaccharides, polyphenolics and metals (1). Normally, proteins in wine are dissolved, (coated in water molecules and not visible). Due to an overall positive charge on the protein at wine pH, they repel one another. The appearance of haze is associated with time, heat, or a change in chemistry that causes previously dissolved proteins to lose their three- dimensional shape, clump together, and come out of solution.
To understand how proteins are behaving in the wine matrix, it is helpful to understand a little bit about the molecular structure of proteins.
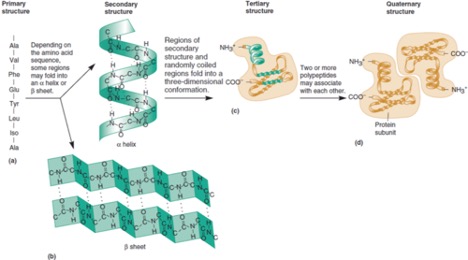
Figure 1: Three dimensional protein folding is determined by the sequence of amino acids. From https://doctorlib.info/genetics/medical-genetics/2.html
Proteins are polymers of amino acids bonded together in a row. In a liquid environment (wine), this string of amino acids folds in on itself again and again, like spaghetti in a bowl. Unlike spaghetti, the strand of amino acids is not the same along the whole length. There are 20 different kinds of amino acids found in living things, and each different kind of amino acid has a different side chain with different chemistry. Amino acid side chains can be positively charged, negatively charged, neutral, large and bulky, small and lean, water loving or water hating. As the string of amino acids folds back on itself, the side chains will interact to attract or repel one another depending on the chemistry of each side chain. The charge of the side chains is very important in folding, as side chains with the same charge will repel one another while side chains with different charges will attract one another. The water-loving nature is also important. The protein will fold in a way to hide the water-hating side chains in the middle of the protein, away from the surface, and put the water-loving side chains on the surface. Based on the sequence of amino acids for each protein (which is determined by genetics) each protein takes on a characteristic folding pattern that is determined by all of the interactions of the side chains (2).
Several changes in the environment can destabilize the folding of the protein (1).
- Acid changes occur in wine through fermentation, malolactic fermentation, cold stabilization (the removal of tartaric acid), blending and aging. A slight acid adjustment (to a lower pH) shifts side chains to a slightly more positive state, so a small addition can help protein stability in the wine a great deal (4).
- If the wine is heated, all of the molecules in the wine start to move faster, and it takes more energy to hold the folding pattern of the protein together. If the interactions were weak to begin with, the protein might unfold. This is the basis of the heat-based stability tests that add a lot of heat to force all of the proteins to unfold. In practice, heat is most likely to occur under non-ideal storage or transport conditions.
- An increase in ethanol (due to fermentation, blending, or fortification) will increase the likelihood that the water-hating side chains in the middle of the protein will come out to the surface. This change in the folding pattern can destabilize the three dimensional structure and cause haze.
- Over time, the protein itself starts to degrade and the amino acid strand breaks apart, leading to unfolding. When the proteins unfold, they are much more likely to interact with one another in solution, and can sometimes form a web, which leads to haze.
- Other components of the wine matrix maybe be involved in preventing protein haze (like mannoproteins) or in participating in the haze (like polysaccharides and phenols). Therefore, changes in the wine due to fining, filtration or additions can change protein stability.
What this means in practice:
Given the above considerations, when testing protein instability to determine a bentonite addition, testing must be done on the final wine. If you are preparing a blend, planning additions or cold stabilization, the protein stability of the wine might change with each of these. If you have to test early, make your bench blend as close to the final wine as possible. If you are planning to add CMC -based products for tartrate stability, it is safest to re-test the stability of the wine prior to addition but after all other operations have been completed. It is also possible to do a bench trial for CMC stability. (If you would like this protocol, feel free to email VaWrex@gmail.com with your request.)
References
(1) Zoecklein, B.; Fugelsang, K. C.; Gump, B. H.; Nury, F. S. Wine Analysis and Production; Springer: New York, 1995.
(2) Urry, L. A.; Cain, M. L.; Wasserman, S. A.; Minorsky, P. V.; Reece, J. B. Campbell Biology (11th Edition); Pearson: New York, 2017.
(3) Jackson, R. S. Wine Science: Principles and Applications, 4 edition.; Academic Press: Amsterdam, 2014.
(4) Zoecklein, B. Protein Stability Determination in Juice and Wine. Virginia Tech Online Enology Publications 1991.
