Varietal Thiols in Wine
Joy Ting
Several WRE experiments in the 2018-2019 season were focused on increasing varietal thiols in aromatic white and rosé wines. These volatile compounds contribute to the varietal character of Virginia wines such as Sauvignon Blanc, Petit Manseng, and Riesling as well as varieties used to produce red and rosé wines. There are several steps in the winemaking process from vineyard to bottle that affect the production, protection and perception of thiols in the finished wine, and therefore several points of intervention for winemakers to consider if they want to increase the impact of these compounds.
Thiols Defined
“Volatile” or “varietal” thiols are a specific class of sulfur containing chemicals present in wine at very low concentrations. (They are measured in ng – that’s 1/1000th of a microgram, which is 1/1000th of a mg!) They were first identified in wine in late 1990’s as those “contributing toward typical aroma of Sauvignon Blanc” (Darriet and Tominaga 1995,Tominaga et al 1996) and, in recent years, have come to be seen as essential to the varietal character of this grape (Benkwitz et al 2012). These are not to be confused with other sulfur-based compounds that contribute to reductive odors and flavors such as cooked leaks, asparagus, and onions. (Another word for thiol is mercaptan. There are several kinds.)
Very high levels of varietal thiols characterize New Zealand Sauvignon Blanc and drive this style of wine (Coetzee 2018 Wines and Vines). Levels in other regions are lower, but are still above the threshold of perception. Varietal thiols have since been reported above the threshold of perception in a number of different varieties including: Gewürtzaminer, Muscat, Riesling, Pinot Gris, Colombard, Chenin blanc, Sauvignon blanc, Sémillon, Petit Manseng, Chardonnay, Merlot, Cabernet Sauvignon, Cabernet Franc, Grenache, Syrah, Cinsault, Mourvèdre, Pinot Noir, and Malbec (Tominaga et al 2000, SARCO lab, Bouchilloux et al 1998). Aromatic analysis of Provencal rosé by Masson and Schneider (2009) identified four molecules responsible for the fruity and amylic notes of these wines; two are thiols.
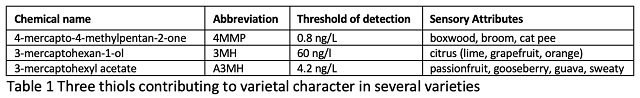
The three varietal thiols that are primarily responsible for the tropical fruity aromas of these wines are shown in Table 1. Each has a very low threshold of detection and can have different perception depending on its concentration. At lower concentrations, 4MMP smells like boxwood and broom, but at high concentrations it smells like cat urine. A3MH is “fruity” at lower concentrations and “sweaty” at high concentrations. In addition to fruit aromas, thiols can also contribute “green” odors such as green pepper and stalky attributes and can enhance the perception of methoxypyrazine (which is also present in Sauvignon Blanc) (Coetzee 2018). Bruce Zoecklein recalled being puzzled at the reduction in perception of methoxypyrazine when first testing microoxygenation, since the measured levels of methoxypyrazine were not decreasing. Eventually the team came to realize that microoxygenation was oxidizing the thiols, which were amplifying the perception of pyrazine (Zoecklein, personal communication).
Concentrations of thiols in aromatic wines can vary widely. For example, 3MH levels have been reported from 29-20,000 ng/L (Coetzee 2018). However the effect of minor differences in thiols concentration has not yet been reported in the literature. One study reported that a difference in 3MH between 0 and 875 ng/L did not greatly affect the aroma of a Sauvignon Blanc (Coetzee 2018), indicating that though these molecules have low sensory thresholds, discrimination among levels may be much less sensitive.
Increasing Thiols
Since the discovery of varietal thiols, many studies have been conducted examining the effect of vineyard and winery practices on their production.
In the Vineyard
In the vineyard, several factors affect production of thiol precursors.
- Water stress: In a study of Bordeaux Sauvignon Blanc, Peyrot des Gachons et al (2005) found that moderate water stress after fruit set increases thiol precursors found in must.
- Nutrient addition: In the same study (Peyrot des Gachons et al 2005), they showed that fruit with adequate nitrogen has significantly higher levels of thiol precursors and that foliar application of nitrogen (just before or after veraison) to make up for deficits resulted in a significant increase in thiol precursors and glutathione. In another study, foliar application of nitrogen and sulfur was shown to increase thiol concentrations 3-12 fold and wines treated this way were shown to be the most aromatically intense by a tasting panel (Lacroux et al 2008). Ammonium nitrate application to soil did not significantly affect thiols. There is a strong correlation between adequate YAN concentration in must and higher 3MH and 3MHS concentrations in wine (Duforcq et al 2009).
- Defoliation of the fruit zone: In a study by Suklje et al (2014), removal of leaves and lateral shoots from the fruit zone at peppercorn size resulted in higher thiols, higher glutathione, and lower methoxypyrazine. Exclusion of UV from the defoliated zone returned thiol to near control levels, indicating an important role for UV radiation in production of thiol precursors.
- Damage to fruit: Botrytis infection (in Sauternes) shows 275 fold increase in thiol precursors in juice and a 12-60 fold increase in 3MH of the finished wine (Sarrazin et l 2007, Thibon et al 2009). This does not mean Botrytis is directly producing thiol precursors. Botrytis infection may trigger a disease resistance pathway in the fruit that leads to thiol formation.
- Harvesting method: Allen et al (2011) found machine harvesting led to higher levels of thiol precursors in the must and varietal thiols in the resulting wine. This may be due to additional time for extraction off skins with broken berries (3MH precursors are mostly localized to skin while 4MMP precursors are found in skin and pulp) or the damage to the berries may trigger a metabolic pathway that produces additional precursors.
In the Cellar
Precursors
Varietal thiols are not present in volatile forms in grapes or juice, but rather as precursors bound to other compounds that must be released by yeast enzymes during fermentation in order to be aromatic.
Several precursor molecules have been identified as potentially important to the production of varietal thiols in wine. Prime candidates include a cysteine conjugate of 3MH, and a glutathione conjugate of 3MH that are present in varying concentrations in must and are resistant to oxidation until cleavage. This means that they are relatively protected at juice stage and only vulnerable after fermentation.
Other candidate precursors include (E)-2-hexanal and other C6 compounds. These are released as anti-microbial compounds when the plant cells are damaged and are toxic to Saccharomyces. During fermentation, the yeast convert the toxic compounds into less toxic compounds by combining them with glutathione (which is present in grape must) to form Glut-3MH. Glut-3MH is a thiol precursor that can then be converted to varietal thiols. C6 compounds can only become thiol precursors if a sulfur containing compound is available, leading to several ideas about way to increase sulfur early in fermentation, including bubbling H2S! (Coetzee 2018).
Even if precursors are present in high concentrations, that is not a guarantee of a highly aromatic wine. When testing 55 different juice/wine pairs Pinu et al (2012) found no correlation between the precursors in juice and the final thiols in wine. Conversion yield of any of these compounds is very poor (0.1% to 12%). Though much remains to be known about the relationship between thiol precursors and final thiol concentrations, several practical steps in winemaking have been shown to be important in thiol production in wines.
Extraction
The first step is to extract as many thiol precursors as possible. Sixty percent of Cys-3MH is localized in grape skins while Cys-4MMP is present in both skin and pulp. Prolonged skin contact coupled with higher fermentation temperature was shown to increase 3MH in Merlot and Cab Sauv (Roland et al 2011). In the same study, harder pressing (2 atm) was shown to extract higher levels of 3MH precursors from Sauvignon Blanc and Melon B grapes, however care must be taken as higher extraction of phenols with high pressure could lead to oxidation later. Skin contact and extraction enzymes are also thought to increase extraction of thiol precursors (Chauffour webinar).
Stabulation of juice lees and enzyme treatment are also thought to increase the extraction of thiol precursors from juice pulp, but with mixed results. The Applied Research Cooperative of Laffort tested the effects of stabulation and enzyme treatment through a multi-site trial including 21 different locations in 2016, including two trials submitted by the WRE. Here only 12 of the 21 wines showed a significant difference between trials and controls in a triangle test, and preferences were split between stabulated and non-stabulated wines. ENose analysis of a subset of these wines done by Bruce Zoecklein showed significant differences while analysis of thiol molecules showed mixed results; of 8 trial wines tested, 5 showed increase in thiols with stabulation, 1 showed a decrease, and 2 had no significant difference between stabulated and non-stabulated wine. Treatment with thiolase enzyme had a larger effect on thiols than stabulation in 3 of 4 wines tested (Laffort, n.d)
Additions
Addition of specialized nutrients may also increase thiol production. Yeast nutrients are routinely added to fermenting must to augment nitrogen for yeast metabolism. Several enological companies have now begun to offer yeast nutrients specially formulated to enhance aromatics of the wine, including formulations to increase thiols. Stimula Sauv Blanc from Scott Labs is formulated to “optimize the uptake of 3MH and 4MMP precursors” as well as stimulate their conversion to thiols in the yeast. AEB sells Fermoplus Tropical with similar description. Three WRE trials were done this year using these products.
In addition to nutrients, Larcher et al (2015) showed the addition of grape tannin containing a significant concentration of S-glutathionylated (GSH-3MH) and S-cysteinylated (Cys-3MH) to fermentation of Meuller-Thurgau and Sauvignon Blanc musts produced wines with higher levels of 3MH and 3MHA. Informal sensory analysis in this study found that the Sauvignon wines produced with the tannin addition were often richer with increased ‘“fruity/green”’ notes, as would be expected with higher thiol concentrations.
Fermentation factors
As mentioned above, yeast are needed to convert thiol precursors to aromatic molecules through action of beta-lyase endopeptidase enzymes. Another enzymes, acetyl-transferase, is needed to convert 3MH to 3MHA. In a study of Bordeaux Sauvignon Blanc, Dubourdieu et al (2006) found variation in the conversion ability between yeast strains. The genetic basis of precursor conversion has been described (Howell et al 2005, Thibon et al 2009) and a family of genes has been identified as responsible. There can be up to 25-fold difference in expression of thiols from one yeast strain to another (BZ, pers comm). Enological companies now market specific yeast strains as “thiol producing”, because they have high number of these genes. Expression of these genes can be turned off by high levels of nitrogen in the juice (Thibon et al 2009), so addition of DAP can decrease thiol release.
Several studies show that fermentation temperature affects thiol release, but guidelines are highly variable (Roland et al 2011). Masneuf-Poumarede et al (2006) tested the effect of temperature and yeast strain on thiol production in Sauvignon Blanc and found temperature had an effect regardless of yeast strain, with higher levels of thiols produced in fermentations conducted at 68F (20 C) compared to 55 F (13 C). In their Sauvignon Blanc style guide, Scottlabs recommends an increase in temperature from 59F (15 C)-65F (18 C) degrees for mineral driven Sauvignon Blanc to 65 (18 C) -72 F (22 C) degrees for tropical Sauv Blanc.
Protection
Once thiols have been produced through fermentation, they must be protected from oxidation because once oxidized, thiols lose their aroma. Thiols are oxidized as part of the following pathway:
- Polyphenoloxidase enzymes in the presence of oxygen convert phenols to quinones.
- Quinones react with copper in the wine to oxidize thiols to non-aromatic forms
Since thiols are present in such small amounts, a small amount of oxidation can cause a big effect on aroma. However, understanding these steps give us several targets for intervention to protect thiols from oxidation:
- Limit oxygen. If oxygen is not present, there is no substrate for enzymes to work. Exclusion of oxygen includes using inert gas in the press, the press pan, and the tank. Judicious use of SO2 will limit oxygen, as well proper topping to limit headspace. Choosing stainless steel over oak also limits oxygen.
- Inactivate polyphenoloxidase (PPO) enzymes. These enzymes come from grapes and are normally present in juice but are usually inactivated by fermentation. Laccase from Botrytis infection is a special type of PPO that is not inactivated by fermentation. Sulfur dioxide at the levels normally used at crush will inhibit normal PPO enzymes but not laccase. If you have Botrytis infected fruit, additional steps are needed. Enzymes can be removed by bentonite and tannin addition during juice settling.
- Limit phenols and metals: There are fining agents that can be used to remove these from finished wine.
- Enhance glutathione: Glutathione binds with the reactive quinone to de-activate it. Bruce Zoecklein stated that glutathione is the single most important component to protecting thiols in finished wine (pers com). Glutathione based additives are available from many companies. In addition, Pons et al (2015) found a significant increase in glutathione extraction from grapes with a few hours of skin contact and pressing under nitrogen
- Use enough SO2. From crusher to bottle, if you want to make a wine rich in thiols, SO2 is one of the best tools you have to protect the wine from oxygen. SO2 will act on several of the above mechanisms including inactivating enzymes, quenching oxidized quinones and limiting free oxygen in the wine.
Aging
Even when bottled, 3MH and 3MHA continue to evolve. Based on reports that the intense tropical fruit aromas of New Zealand Sauv Blanc diminished rapidly in the bottle, Herbst-Johnstone et al (2011) monitored the evolution of 3MH and 3MHA as well as levels of glutathione and SO2 in bottled wine stored in the dark at 15 degrees C for 7 months. They found 3MHA (passionfruit, guava) was the least stable, declining steadily. 3MH decreased only slightly in the first 3 months, then increased in the last 4 months. The authors hypothesize 3MHA is hydrolyzing back to 3MH during this time. Glutathione concentrations also declined to the point they were no longer protecting the thiols after the first year while SO2 remained stable.
Summary
Production of wine with high tropical fruit aromas requires care in all steps of the winemaking from the vineyard to the bottle. The molecules responsible for this style of wine are present in very small concentrations, have low perception thresholds, and are vulnerable to loss due to oxidation. Though the mechanism of their production is not yet fully understood, some tools are available for the vigneron to make informed choices to produce the style of choice:
- Proper nutrition in the vineyard
- Defoliation and UV exposure in the fruit zone
- Activating grape stress responses through Botrytis, machine harvesting or skin contact
- Extraction from skins through skin contact or stabulation
- Specialized yeast nutrients and yeast strains to optimize precursor conversion
- Limiting oxidation by limiting oxygen, enzymes, phenols
- Enhancing protection by using SO2 and gluathione
References
- Allen, T., M. Herbst-Johnstone, M. Girault, P. Butler, G. Logan, S. Jouanneau, L. Nicolau and P.A. Kilmartin. (2011) "Influence of grape-harvesting steps on varietal thiol aromas in sauvignon Blanc wines." J. Agric. Food Chem. 59 (19), 10641-10650.
- Bouchilloux, P., P. Darriet, R. Henry, V. Lavigne-Cruege, D. Dubourdieu. (1998) “Identification of Volatile and Powerful Odorous Thiols in Bordeaux Red Wine Varieties”. J. Agric. Food Chem., 46 (8), pp 3095–3099.
- Chaufour, E. (2019) “Thiols and Beyond: Aromatic expressions in wines”[Webinar]. Bucher Vaslin North America. Accessed January, 2019. https://www.bvnorthamerica.com/webinars
- Coetzee, C. (2018) “Grape-Derived Fruity Volatile Thiols.” Wines & Vines. April 2018 issue. Accessed December 17, 2018. http://www.winesandvines.com/features/197002.
- Darriet, P., T. Tominaga, V. Lavigne, J. Boidron, & D. Dubourdieu. (1995) Identification of a powerful aromatic component of Vitis vinifera L. var. Sauvignon wines 4-mercapto-4-methylpentan- 2-one. Flavour and Fragrance Journal, 10(6), 385-392.
- Dubourdieu, D., T. Tominaga, I. Masneuf, C. Peyrot des Gachons, and M.L. Murat. (2006) “The Role of Yeasts in Grape Flavor Development during Fermentation: The Example of Sauvignon Blanc.” American Journal of Enology and Viticulture 57(1) 81–88.
- Helwi, P., C. Thibon, A. Habran, G.Hilbert, S. Guillaumie, S. Delrot, P. Darriet, and C. Van Leeuwen. (2015) “Effect of Vine Nitrogen Status, Grapevine Variety and Rootstock on the Levels of Berry S-Glutathionylated and S-Cysteinylated Precursors of 3-Sulfanylhexan-1-Ol.” OENO One 49(4): 253.
- Herbst-Johnstone, M., L.Nicolau, and P. A. Kilmartin. (2011) “Stability of Varietal Thiols in Commercial Sauvignon Blanc Wines.” American Journal of Enology and Viticulture 62(4): 495–502.
- Howell, K. M. Klein, J.H. Swiegers, Y. Hayasaka, G.M. Elsey, G. H. Fleet, P. B. Høj, I.S. Pretorius, and M.A. de Barros Lopes. (2005) “Genetic Determinants of Volatile-Thiol Release by Saccharomyces cerevisiae during Wine Fermentation.” Appl Environ Microbiol. 71(9):5420-6.
- Lacroux, F., O. Tregoat, C. Van Leeuwen, A. Pons, T. Tominaga, V. Lavigne-Cruege, D. Dubourdieu. (2008). Effect of foliar nitrogen and sulphur application on aromatic expression of vitis vinifera L. cv. Sauvignon Blanc. Journal International des Sciences de la Vigne et du Vin, 42(3), 1-8.
- Larcher, R, L. Tonidandel, T. Román Villegas, T. Nardin, B. Fedrizzi, and G. Nicolini. (2015) “Pre-Fermentation Addition of Grape Tannin Increases the Varietal Thiols Content in Wine.” Food Chemistry 166: 56–61.
- Masneuf-Pomarède, I., C. Mansour, M. Murat, T. Tominaga, and D. Dubourdieu. (2006) “Influence of Fermentation Temperature on Volatile Thiols Concentrations in Sauvignon Blanc Wines.” International Journal of Food Microbiology 108(3): 385–90.
- Masson, G., and R. Schneider. (2009) “Key Compounds of Provence Rosé Wine Flavor.” American Journal of Enology and Viticulture 60(1):116–22.
- Pinu, F. R., S. Jouanneau, L. Nicolau, R.C. Gardner, and S. G. Villas-Boas. (2012) “Concentrations of the Volatile Thiol 3-Mercaptohexanol in Sauvignon Blanc Wines: No Correlation with Juice Precursors.” American Journal of Enology and Viticulture 63(3): 407–12.
- Pons, A., V. Lavigne, P. Darriet, and D. Dubourdieu. (2015) “Glutathione Preservation during Winemaking with Vitis Vinifera White Varieties: Example of Sauvignon Blanc Grapes.” American Journal of Enology and Viticulture 66(2): 187–94.
- “Report | ARC by Laffort.” Accessed December 13, 2018. https://arcbylaffort.com/stabulation-report/.
- Roland, A., R. Schneider, A. Razungles, and F. Cavelier.(2011) Varietal Thiols in Wine: Discovery, Analysis and Applications. Chemical Reviews 111:7355–7376.
- Peyrot des Gachons, C., C. Leeuwen, T. Tominaga, J. Soyer, J. Gaudillere, D Dubourdieu. (2005) Influence of water and nitrogen deficit on fruit ripening and aroma potential of vitis vinifera L cv Sauvignon Blanc in field conditions. Journal of the Science of Food and agriculture, 85, 73-85.
- Sarrazin, E., S. Shinkaruk, T. Tominaga, B. Bennetau, E. Frerot, D. Dubordieu. (2007) Odorous impact of volatile thiols on the aroma of young botrytized sweet wines: Identification and quantification of new sulfanyl alcohols. Journal of Agricultural and Food Chemistry, 55:1437-1444.
- Šuklje, K., G. Antalick, Z. Coetzee, L.M. Schmidtke, H. Baša Česnik, J. Brandt, W.J. du Toit, K. Lisjak, and A. Deloire. (2014) “Effect of Leaf Removal and Ultraviolet Radiation on the Composition and Sensory Perception of Vitis Vinifera L. Cv. Sauvignon Blanc Wine: Light, Ultraviolet Radiation and Wine Composition.” Australian Journal of Grape and Wine Research 20(2): 223–33.
- Thibon, C.,P. Marullo, O. Claisse, C. Cullin, D. Dubourdieu &T. Tominaga. (2008) “Nitrogen catabolic repression controls the release of volatile thiols by Saccharomyces cerevisiae during wine fermentation.” FEMS Yeast Res. 8(7):1076-86.
- Tominaga, T., P. Darriet, and D. Dubourdieu. (1996) “Identification de I'acetate de 3-mercaptohexanol, compos~ & forte odeur de buis, intervenant dans I'ar6me des vins de Sauvignon.” Vitis 35:207-210.
- Tominaga, T., R. Baltenweck-Guyot, C. Peyrot Des Gachons, and D. Dubourdieu. (2000) “Contribution of Volatile Thiols to the Aromas of White Wines Made From Several Vitis Vinifera Grape Varieties.” American Journal of Enology and Viticulture 51(2): 178–81.
