Using chitosan (Enartis Stab Micro M) to minimize spoilage during ambient fermentations (2021)
Todd Henkle
The Vineyards and Winery at Lost Creek
Summary
At the Vineyards and Winery at Lost Creek, red wines have been fermented without the addition of commercial yeast since 2016. They believe that greater diversity in the microbial population during ambient fermentation leads to more complex finished wines. However, ambient fermentation also includes a greater level of risk for elevated levels of acetic acid and ethyl acetate due to the activity of non-Saccharomyces yeast and bacteria. In this experiment, Stab Micro M, an antimicrobial chitosan product, was used to limit the activity of spoilage yeast and bacteria. Chitosan use had no effect on the kinetics of fermentation. Wines treated with chitosan completed fermentation and aging with lower levels of acetic acid (0.76g/L vs. 0.92 g/L) and comparable levels of ethyl acetate (104 mg/L and 113 mg/L). The wines were not different in a triangle test. Any differences in volatile acidity were below threshold of detection. Chitosan had no effect on sensory scores for complexity.
Introduction
At the Vineyards and Winery at Lost Creek, red wines have been fermented without the addition of commercial yeast since 2016. They believe that greater diversity in the microbial population during ambient fermentation leads to more complex finished wines. However, ambient fermentation also includes a greater level of risk for elevated levels of acetic acid and ethyl acetate due to the activity of non-Saccharomyces yeast and bacteria. The purpose of this experiment was to explore the use of Stab Micro M, an anti-microbial chitosan product manufactured by Enartis specifically for use during fermentation, with specific attention to effects on volatile acidity and complexity in ambient fermented wines.
At Lost Creek, fruit is processed cold, then placed in the cellar for a slow warm up, allowing a de-facto cold soak of approximately 3 days prior to the start of robust fermentation. One effect of cold soak is the activity of non-Saccaromyces yeast. These yeast may have a positive impact on aroma and flavor complexity by releasing flavor precursors that Saccharomyces later transform. They can also increase fruity aromas through the production of significant amounts of esters1. However, they may also have negative sensory effects. Hanseniaspora (AKA Klockera) is the main species found on mature grapes, which can produce positive compounds as described above, however this cryotolerant yeast can also produce large amounts of ethyl acetate and acetic acid2,3. For a broader discussion of non-Saccharomyces yeast, see the Learn section of the WRE website.
One approach to limiting spoilage organisms is the use of Stab Micro M, a chitosan-based product developed specifically for use during fermentation. Chitosan binds in different degrees to different microbes based on the chemical properties of the cell wall itself, with high degree of correlation to the hydrophilicity of the wall4. Chitosan has been shown to have some efficacy against a wide range of grapevine and wine microbes including downy mildew5, powdery mildew6, Phomopsis7, Lactobacillus, Oenococcus, and Brettanomyces4. Gram negative bacteria are more susceptible to binding than gram positive4. Due to its versatility as an antimicrobial agent, chitosan in various forms has been used worldwide at nearly every stage of wine production including vineyard applications, on grapes during transport and storage, at crush, after fermentation and during the aging of wine8–10.
Most chitosan products are recommended for use in finished wine. Stab Micro M (Enartis) is a chitosan-based product specially formulated for use on juice and must to reduce the activity of a wide range of microbes, leading to reduction in volatile acidity, sulfide defects, volatile phenols and production of other off-flavors. It can be used in conjunction with SO2 or as a replacement, depending on the condition of grapes and preference of the winemaker8. Due to its anti-microbial effects, chitosan may decrease the production of volatile acidity, but may also reduce complexity in the wine. Also, due to the large populations of microbes in grape must, chitosan at its recommended dose may have no effect at all.
A previous experiment at Lost Creek in 2020 included chitosan treatment in a cold soak experiment, with no measurable effects. However, the dose rate of Stab Micro M (3 g/hL) was below the manufacturer’s recommended dose rate of 10-40 g/hL. The purpose of this experiment was to determine if using Stab Micro M at 25 g/hL led to differences in complexity or spoilage characteristics in the finished wine.
Methods
Cabernet Franc was harvested from Williams Gap vineyard on 9/30. Fruit was chilled overnight before processing into TBins. Each bin received 0.98 tons of fruit for an estimated 627 liters of wine (650 tons/Liter). Fruit included 15% whole clusters, 35% whole berries and 50% crushed berries. At crush, one bin received 50 ppm SO2 only while the other received 50 ppm SO2 and 20 g/hL Stab Micro M (chitosan). After processing, bins were drained of 12% of the volume (78 L), then gassed thoroughly, wrapped in plastic and moved to the cellar to allow for the buildup of ambient yeast.
On 10/4, juice was taken for analysis. To sample for juice microbiology, a representative juice sample was taken after homogenization (punchdown) but before inoculation. The juice was centrifuged to form a pellet. Supernatant juice was poured into a new tube, leaving a small amount of juice on the pellet. Both tubes were capped and shipped overnight to ETS without freezing (which would kill the cells). Upon arrival, ETS reconstituted the sample before testing to maintain proper concentrations.
Based on in-house pH values, 3 g/L tartaric acid was added to each bin. Bins were lightly punched down and gassed until signs of fermentation began, after which they received three punchdowns daily. Fermentations were checked for Brix and temperature once daily. At the completion of fermentation, bins were gassed each day before pressing on 10/24 for a total of 23 days of maceration. After pressing, wine was allowed to settle, then transferred to comparable oak barrels for malolactic fermentation. Wine was treated with 70 ppm SO2 on 12/3, at the completion of malolactic fermentation.
Sensory analysis was completed by a panel of 20 wine producers. Wines were presented blind in randomly numbered glasses. Tasters were presented with three wines, two of one type and one of another, and asked to identify which wine was different (a triangle test). There were three tasting groups with the unique wine in the triangle test balanced between groups. Tasters were then asked to score each wine on a scale of 0 to 10 for aromatic intensity, fruit intensity, volume/body, complexity, and perception of acidity. They were also given open ended questions to describe the wines. Results for the triangle test were analyzed using a one-tailed Z test. Descriptive scores were analyzed using repeated measures ANOVA.
Results
Cabernet Franc fruit was very similar at processing between the two lots (Table 1). Chitosan treatment did not affect the pace or temperature of fermentation (Figure 1). Both lots completed malolactic fermentation with similar chemistry (Table 2). The barrels containing wine treated with chitosan during fermentation had lower levels of acetic acid at the end of malolactic fermentation (February) as well as after aging (April)(Table 3). Ethyl acetate values for both treatments were very near the level of detection (90-150 mg/L).
The microbial community was sampled at juice and after wine aging (Table 4). The bin that was treated with chitosan started with higher values for several Lactobacillacea as well as Saccharomyces. In the finished wine, the wine treated with Stab Micro M had slightly lower values for Brettanomyces, Lactobacillus, and Oenococcus. However, with the exception of Oenococcus, these values were very low in both treatments.
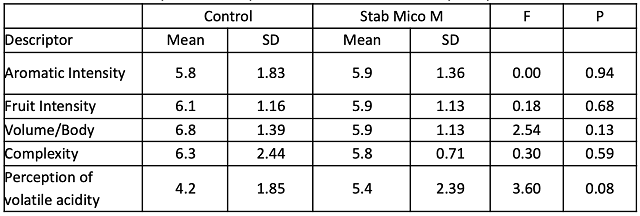
In a triangle test, 8 out of 20 respondents were able to distinguish which wine was different, indicating the wines were not significantly different (Z= 0.40, p= 0.35). There were no significant differences in scores for aromatic intensity, fruit intensity, volume/body, complexity or perception of volatile acidity (Table 5). Though differences in volatile acidity were not perceptible at this time, these wines will age for another year, during which time differences in acetic acid may become more apparent.
Table 1: Juice chemistry for control and chitosan treated Cabernet Franc (in-house data)
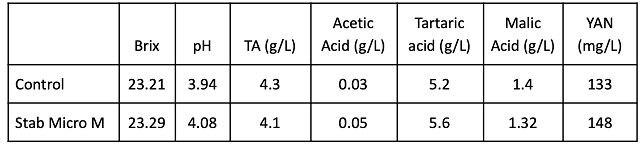
Figure 1: Fermentation kinetics for control and chitosan treated Cabernet Franc (in-house data)
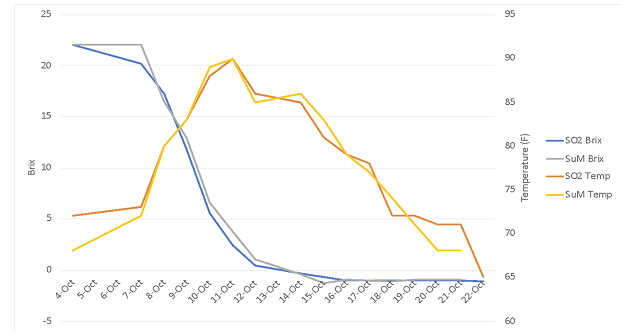
Table 2: Post-malolactic fermentation chemistry for control and chitosan treated Cabernet Franc (Feb 28, ICV labs)
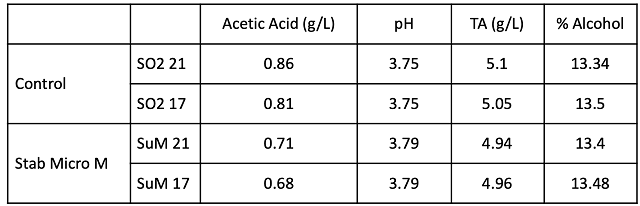
Table 3: Comparison of Volatile Acidity for Feb and April sampling (ICV Labs)
Table 4: Comparison of microbes in juice and wine for two treatments of Cabernet Franc
(ETS Labs)
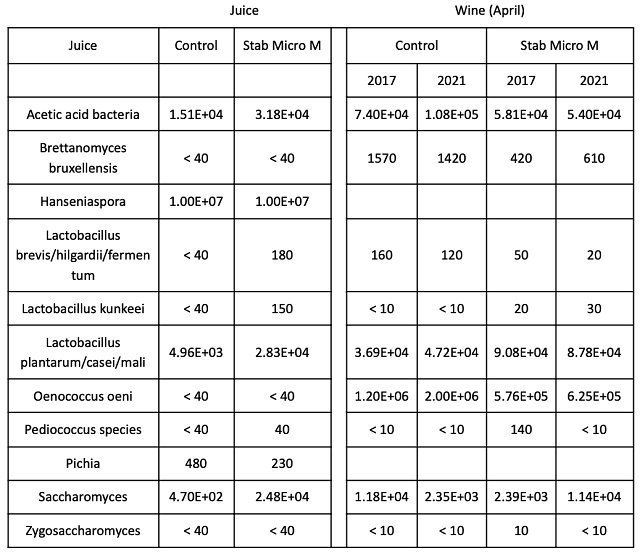
Table 5: Statistical analysis for descriptive scores from blind sensory analysis of Cabernet Franc
References
(1) Razungles, A. Extraction Technologies and Wine Quality. In Managing Wine Quality: Volume 2 – oenology and wine quality; Woodhead Publishing: Cambridge, 2010; Vol. 2.
(2) Jackson, R. S. Wine Science: Principles and Applications, 4 edition.; Academic Press: Amsterdam, 2014.
(3) Diversity of Wine Yeasts | Viticulture and Enology. https://wineserver.ucdavis.edu/industry-info/enology/wine-microbiology/yeast-mold/diversity-wine-yeasts (accessed 2018-11-13).
(4) Chung, Y.; Su, Y.; Chen, C.; Jia, G.; Wang, H.; Wu, J. C. G.; Lin, J. Relationship between Antibacterial Activity of Chitosan and Surface Characteristics of Cell Wall. Acta Pharmacol Sin 2004, 5.
(5) Romanazzi, G.; Mancini, V.; Feliziani, E.; Servili, A.; Endeshaw, S.; Neri, D. Impact of Alternative Fungicides on Grape Downy Mildew Control and Vine Growth and Development. Plant Disease 2016, 100 (4), 739–748. https://doi.org/10.1094/PDIS-05-15-0564-RE.
(6) Iriti, M.; Vitalini, S.; Di Tommaso, G.; D’Amico, S.; Borgo, M.; Faoro, F. New Chitosan Formulation Prevents Grapevine Powdery Mildew Infection and Improves Polyphenol Content and Free Radical Scavenging Activity of Grape and Wine: Grape Powdery Mildew Control by Chitosan. Australian Journal of Grape and Wine Research 2011, 17 (2), 263–269.
(7) Nascimento, T.; Rego, C.; Oliveira, H. Potential Use of Chitosan in the Control of Grapevine Trunk Diseases. Phytopathologia Mediterranea 2007, 46 (2), 8.
(8) Barrett, L. Microbial Stability and Control: EnartisStab Micro (Chitosan) Application during Wine Maturation, 2019.
(9) Singh, R. K.; Martins, V.; Soares, B.; Castro, I.; Falco, V. Chitosan Application in Vineyards (Vitis Vinifera L. Cv. Tinto Cão) Induces Accumulation of Anthocyanins and Other Phenolics in Berries, Mediated by Modifications in the Transcription of Secondary Metabolism Genes. IJMS 2020, 21 (1), 306.
(10) Singh; Soares; Goufo; Castro; Cosme; Pinto-Sintra; Inês; Oliveira; Falco. Chitosan Upregulates the Genes of the ROS Pathway and Enhances the Antioxidant Potential of Grape (Vitis Vinifera L. ‘Touriga Franca’ and ’Tinto Cão’) Tissues. Antioxidants 2019, 8 (11), 525.
