Exploring nutrient strategies in red wine fermentations – creating a nutrient desert (2020)
Michael Heny
Michael Shaps Wineworks
Summary
In his popular book “Postmodern Winemaking”, Clark Smith describes the concept of leaving a nutrient desert at the end of fermentation as part of an integrated Brett management strategy. However, yeast need adequate nutrition to complete fermentation, leading to questions of how much to supplement nitrogen without adding too much. In this experiment, both control and treatment lots of Tannat received the amount of Superfood recommended by the manufacturer. The control also received DAP while the treatment did not. There were not large differences in fermentation kinetics or finished wine chemistry based on the differences in additions. The wines were significantly different in a triangle test, with the wine that received DAP supplementation receiving significantly higher scores for body/volume.
Introduction
The purpose of this experiment was to explore effects of not adding DAP to a red wine fermentation. At Wineworks, the standard protocol for nutrients is to measure YAN enzymatically (with NOPA and Ammonia kits, Unitech Scientific) after grape processing, then determine nutrient additions based on the manufacturer’s recommendations for Superfood and DAP1. However, a recent analysis of sparkling wine base found higher than desired nutrients after lees aging, leading to the concern that ample nutrient addition during fermentation may be providing nutrients for spoilage organisms later. At Wineworks, Tannat ages for 20 months prior to bottling, allowing plenty of time for spoilage organisms to work.
More generally, the question of nutrient addition is complicated. In his popular book “Postmodern Winemaking”, Clark Smith2 describes the concept of leaving a nutrient desert at the end of fermentation as part of an integrated Brett management strategy. The idea here is to deliver fruit from the vineyard with enough nutrients to complete fermentation without leaving extra nutrients for spoilage organisms to feed on during aging2. He does not address how to approach nutrient supplementation in the winery in the case of nitrogen deficiency, but one can extend his logic to limit supplementation to what is minimally needed. Yet, caution should be taken as textbooks and enology literature suggest that nutrient limitation can lead to cessation of sugar transport, shutting down of glycolysis, production of sulfur-like off odors and loss of aroma and flavor complexity3–5.
These questions are further complicated by differences in nutrients themselves. Nitrogen supplementation is most commonly accomplished by the addition of complex nutrients (formulations of yeast autolysis products containing amino acids and peptides) and inorganic nitrogen (DAP). There are known differences in nutrient uptake and utilization between these sources3. Superfood is a complex nutrient that includes autolyzed yeast products, micronutrients such as biotin and thiamine, as well as 32.5% DAP. However, YAN deficient musts often require more nitrogen supplementation than can be accomplished by Superfood alone, leading to supplemental additions of DAP1. The purpose of this experiment was to determine the kinetic and sensory effects of neglecting the addition of DAP during nutrient supplementation.
Methods
Fruit was hand harvested, destemmed and lightly crushed into TBins with the addition of 30 ppm SO2, 0.09 mL/L Color Pro (Scottlabs), 3.5 g/L Nadalie FMT+ cubes, and 0.37 g/L Tanin VR Supra (Laffort). Nitrogen status of the must was measured in-house using enzymatic kis (Unitech Scientific) after processing and before inoculation. Must was inoculated with 0.2 g/L BM45 yeast rehydrated in 0.15 g/L GoFerm the day after processing. Bins were punched down twice daily with the addition of 20 g/L sugar and nutrients four days after inoculation. The control (DAP) bin received 0.48 g/L of Superfood and 0.15 g/L of DAP. The treatment (no DAP) bin received 0.48 g/L Superfood only.
Bins were monitored daily for Brix depletion and temperature and pressed at the completion of alcoholic fermentation. Wine was allowed to settle for two days before transfer to barrel. Wine was inoculated for malolactic fermentation with 0.01 g/L Enoferm Alpha (Scottlabs). At the completion of fermentation, 50 ppm SO2 was added.
Sensory analysis was completed by a panel of 33 wine producers. Due to restrictions put in place during COVID-19, sensory analysis was completed using shipped samples. Each wine producer received three wines in identical bottles, filled on the same day, each coded with random numbers. Two of the bottles contained the same wine while the third bottle contained the different wine. Participants were asked to identify which wine was different (a triangle test). There were four tasting groups with the unique wine in the triangle test balanced among the groups. Participants were then asked to score each wine on a scale of 0 to 10 for overall aromatic intensity, fruit intensity, and body/volume. They were also given open ended questions to describe the wines. Results for the triangle test were analyzed using a one-tailed Z test. Descriptive scores were analyzed using repeated measures ANOVA.
Results
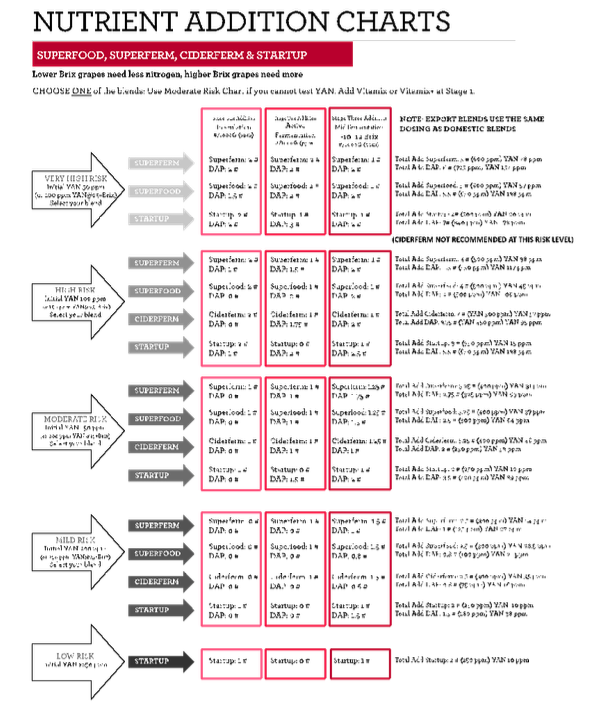
Appendix A reprints the guidelines for Superfood addition given by the manufacturer (BSG). In this case, must was chaptalized to a target of 21.4°Brix and had a YAN of 170 mg/L (Table 1). Based on recommendations from the manufacturer, a target YAN of 250ppm was set, and the must was determined to be at “mild risk”. In this case, only a small amount of DAP was called for (0.15 g/L). Though the manufacturer recommends two nutrient additions, the pace of fermentation in red wine at Wineworks is usually such that one addition is made so as not to miss the window of opportunity for the second addition. Nutrient additions were made during “stage 2”, when fermentation was fully underway with active bubbling and a cap had formed, after Brix depletion of 3-5°Bx.
There were no large differences in finished wine chemistry between lots (Table 2), however the lot with no DAP finished fermentation with lower alcohol and higher pH than the lot that received full nutrition. Neither nutrient strategy led to large amounts of leftover nitrogen (Table 3). Both fermentations progressed steadily without difficulty (Figure 1) and completed fermentation with <1.0 g/L residual sugar (ICV labs, data not shown). The fermentation that received DAP also has slightly higher color intensity (Figure 2). These wines had 22ppm and 25ppm SO2 at the time of analysis, indicating this difference is not likely due to differences in SO2, however the difference may reflect differences in pH.
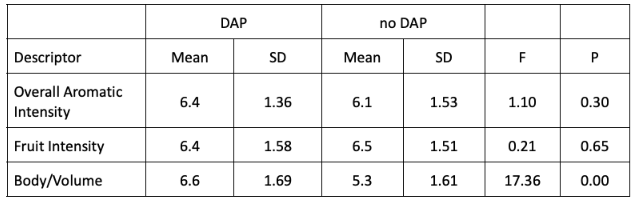
In a triangle test, 17 out of 33 respondents were able to distinguish which wine was different, indicating the wines were significantly different (Z=2.03, p= 0.02). There were no significant differences in scores for overall aromatic intensity or fruit intensity. The wine that received DAP addition scored significantly higher for body/volume (Table 4).
References
(1) BSG Nutrient Addition Charts. BSG.
(2) Smith, C. Postmodern Winemaking: Rethinking the Modern Science of an Ancient Craft by Clark Smith (7-Jan-2014) Hardcover; University of California Press, 2014.
(3) Jackson, R. S. Wine Science: Principles and Applications, 4 edition.; Academic Press: Amsterdam, 2014.
(4) Bisson, L. F. Stuck and Sluggish Fermentations. American Journal of Enology and Viticulture 1999, 50 (1), 107–119.
(5) Bisson, L. F.; Butzke, C. E. Diagnosis and Rectification of Stuck and Sluggish Fermentations. Am J Enol Vitic. 2000, 51 (2), 168–177.
Table 1: Fruit chemistry after crush (in-house labs)

Table 2: Wine chemistry for two treatments of Tannat (ICV Labs)
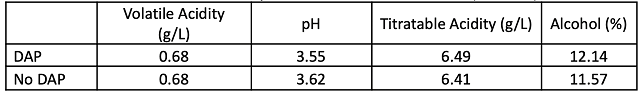
Table 3: Nitrogen chemistry for two treatments of Tannat (mg/L) (Virginia Tech Enology Services Lab)
Figure 1: Fermentation kinetics for two treatments of Tannat (in-house data)
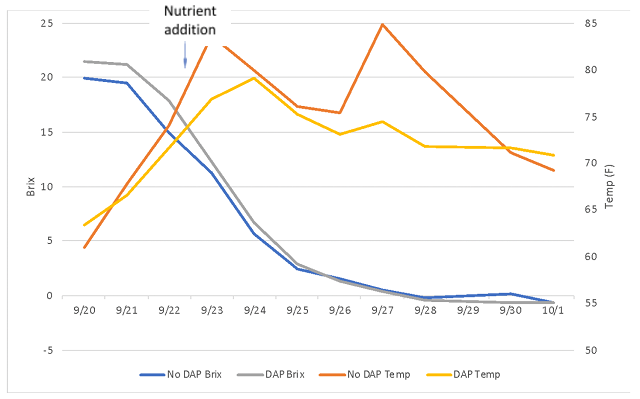
Figure 2: Color metrics for two treatments of Tannat (ICV labs)
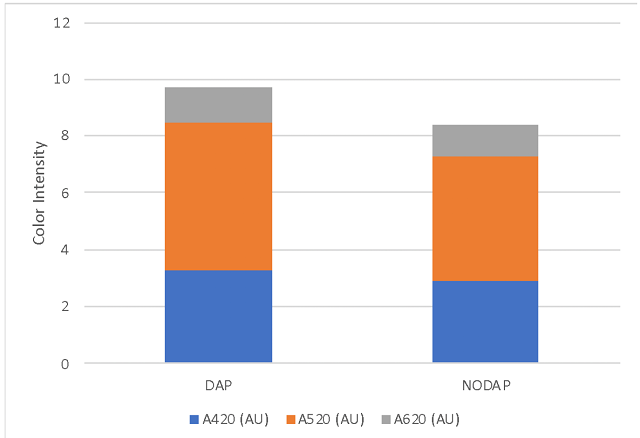
Table 4: Statistical analysis for descriptive scores from blind sensory analysis of two treatments of Tannat
Appendix A: Manufacturer’s recommendations for Superfood and DAP addition (BSG catalogue)