Why we measure SO2
Joy Ting
January 2022
Sulfur dioxide is a traditional, inexpensive additive used widely at many different stages of modern wine making to combat oxidation and microbial spoilage. It can be used pre-fermentation to control microbial populations that come in on grapes and the oxidation of juice, post-fermentation to protect wine from oxidation and spoilage during aging, for storage of barrels to prevent microbial spoilage, and even as a general cleaning agent in the winery (sprays on surfaces, etc…).
When used in the winery, SO2 is most often in a liquid form. When SO2 dissolves in water, it doesn’t remain only as SO2. Instead, it interacts with the water in a way that takes three forms: molecular sulfur, bisulfite and sulfite. Figure 1 gives a graphical representation of the relationship between pH and the forms of sulfur dioxide. Between pH of 3 and 4, which describes most wine, bisulfite is the predominant form of sulfur dioxide while molecular sulfur and sulfite are scarce1. The balance of forms is important because not all forms of sulfur dioxide have the same activity.
Figure 1: Forms of sulfur dioxide in solution are pH dependent. Image from Rotter n.d2.

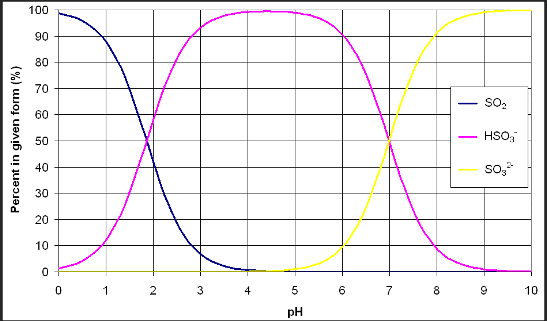
Molecular sulfur (SO2) is prized because of its antimicrobial activities. As the only form of sulfur dioxide that is not charged, molecular sulfur can penetrate the cell membranes of microbes and cause cellular damage and death.1,3,4 Molecular SO2 is also volatile, which means this is the form that can cause negative sensory aromas in the headspace of wine and can be lost during aging as it diffuses into the headspace of barrels and dissipates1. The volatility of molecular SO2 is also the basis for separation from the wine matrix during aeration oxidation testing, and is the form detected in the headspace gas detection tube method of analysis.
The bisulfite form of sulfur dioxide (HSO3-) dominates at wine pH. Bisulfite is a potent inhibitor of enzymes that causes enzymatic browning in juice and wine4. However, bisulfite binds to many constituents in the wine including acetaldehyde, anthocyanins, and sugars1,3,4. Once bound, it is no longer active as an antioxidant. This binding is not irreversible, however. Under very acidic conditions and high heat, bisulfite is released. This is the basis of total SO2 testing, but can be erroneously counted as free when acid is used during free SO2 testing such as aeration oxidation and Ripper titration.
SO2 testing is one of the most common analytical tests performed in wineries, with addition rates relying on the results of these tests. Most wineries test for both free and total sulfur dioxide. Free SO2 refers to any form of SO2 in wine that is not bound to another molecule and gives an indication of the antimicrobial and antioxidant protection conveyed to the wine. Total sulfur includes both the free and bound fractions. The legal limit for total SO2 is 350 ppm, however most wineries limit total SO2 to 200 ppm for sensory reasons4,5. Free SO2 targets depend on the antimicrobial and antioxidant goals. A general rule of thumb is to maintain a molecular SO2 between 0.5 and 0.8 mg/L for antimicrobial properties6,7. It is also recommended to keep free SO2 above 10 ppm in red wines and 20 ppm in white wines to avoid oxidation3. Because the bisulfite form of SO2 binds readily to many common constituents of wine, free SO2 cannot be calculated from SO2 additions but rather must be measured. There are several methods currently in use for measuring free SO2. The principles behind these tests and potential problems with each test are the topic of the remainder of this newsletter. For more detail into the chemistry of SO2, target SO2 levels and management strategies, see the July 2020 Newsletter on SO2 Management.
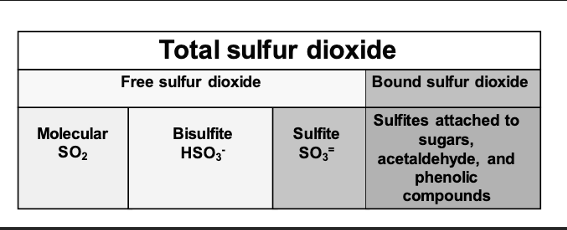
Figure 2: Forms of free and bound sulfur dioxide in wine. From Zoecklein8
References
(1) Boulton, R.; Singleton, V. L.; Bisson, L. F.; Kunkee, R. E. Principles and Practices in Winemaking; Chapman and Hall, Inc: New York, 1996.
(2) Rotter, B. Sulfur Dioxide. Improved Winemaking: advanced theory, practical solutions and opinions.
(3) Ribereau-Gayon, P.; Dubourdieu, D.; Doneche, B.; Lonvaud, A. Handbook of Enology Volume 1: The Microbiology of Wine and Vinifications, 2nd ed.; John Wiley & Sons: West Sussex, England, 2006.
(4) Zoecklein, D. B. Sulfur Dioxide (SO2). Enology Notes Downloads, 16.
(5) Stamp, C. How Much SO2 to Add and When. Wines and Vines 2011.
(6) Margalit, Y. Concepts in Wine Chemistry, 3rd ed.; The Wine Appreciation Guild LTD: San Francisco, California, 2012.
(7) Cojocaru, G.; Antoce, O. Chemical and Biochemical Mechanisms of Preservatives Used in Wine: A Review. Sci. Pap. Ser. B: Hortic. 2015, 56, 457–466.
(8) Zoecklein, B. W. Sulfur Dioxide: Science behind This Antimicrobial, Antioxidant, Wine Additive. Practical Winery and Vineyard Journal 2009.
