The Impact of the timing and amount of SO2 addition on Cab Franc Chemistry, Structure and Sensory Characteristics (2018)
Kirsty Harmon
Blenheim Vineyards
Summary
Sulfur dioxide is a traditional, inexpensive additive used widely at many different stages of modern wine making to combat oxidation and microbial spoilage. Despite all that is known about the chemistry and interactions of SO2 in wine, many practical questions remain for winemakers. In this study, a single lot of Cabernet Franc wine was divided into six treatments with varying barrel age, dose and timing of SO2 addition. The free and total SO2, SO2 addition rates, volatile acidity and microbiological evolution are reported. High initial dose led to higher total sulfur in each case. However, wines that received a high initial dose were also closer to the target SO2 rate for aging for a higher proportion of the aging period. High initial dose may have helped preserve anthocyanins. Wines receiving high initial doses had lower overall accumulation of acetic acid during aging. New barrels had lower free SO2 than their neutral counterparts on the same SO2 schedule. They accumulated higher amounts of acetic acid and had high microbial populations of Pediococcus and acetic acid bacteria. If using new barrels, care should be taken to monitor SO2 levels early in aging to accommodate for additional SO2 binding by new barrels vs. older barrels. Delaying SO2 addition by two weeks decreased total sulfur levels for both high and low dose wines. These wines began with higher acetic acid but experienced very little acetic acid accumulation during aging. The high, delayed dose was closest of all of the wines to the target SO2 during aging. Though these wines were distinguishable from one another during sensory analysis, there was no significant preference for one wine over the other and no consistent trend in the differences (color, astringency, fruit or aromatic intensity).
Introduction
Sulfur dioxide is a traditional, inexpensive additive used widely at many different stages of modern wine making to combat oxidation and microbial spoilage. It can be used pre-fermentation to control microbial populations that come in on grapes and oxidation of juice, post-fermentation to protect wine from oxidation and spoilage during aging, for storage of barrels to prevent microbial spoilage, and even as a general cleaning agent in the winery (sprays on surfaces, etc…). Despite its widespread use, many questions of everyday practical use remain, including the best timing and magnitude of SO2 additions. The following experiments focus on the use of SO2 post-fermentation, during aging of Cabernet Franc wine.
When used in the winery, SO2 is most often in a liquid form. When SO2 dissolves in water, it doesn’t remain only as SO2. Instead, it interacts with the water in a way that takes three forms: molecular sulfur, bisulfite and sulfite (Figure 1). How much of any one form is present depends on the pH of the solution, with the equation shifted to the left in low pH solutions and to the right in high pH solutions, meaning, the higher the pH, the less molecular sulfur dioxide is available1–3. Molecular sulfur is prized because of its antimicrobial activities. As the only form of sulfur dioxide that is not charged, molecular sulfur can penetrate the cell membranes of microbes and cause cellular damage and death. Once inside the cells of yeast and bacteria, which themselves have a pH of 6.5, molecular sulfur converts to the bisulfite form and denatures proteins, disrupts cell membranes, and ends cell functioning1–3. The bisulfite form of sulfur dioxide dominates in wine pH. Bisulfite is a potent inhibitor of enzymes such as tyrosinase (aka polyphenoloxidase) that causes enzymatic browning in juice and wine, though it is somewhat less effective against laccase, the oxidative enzyme produced by Botrytis2. However, the activity of bisulfite is constrained by the fact that it binds many constituents in the wine including acetaldehyde, anthocyanins, and sugars1–3. Once bound, it is no longer active as an antioxidant. At the pH of wine, the sulfite ion is nearly non-existent.
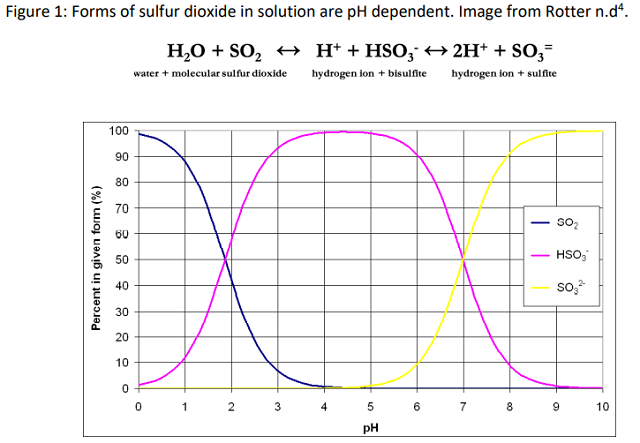
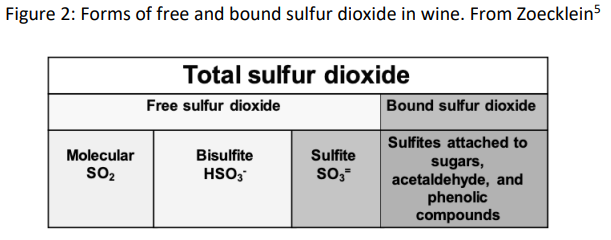
When managing SO2 in wine production, free and total sulfur dioxide are the most common measurements used. Free SO2 refers to any form of SO2 in wine that is not bound to another molecule. As mentioned above, the bisulfite form of SO2 binds readily to many common constituents of wine, which also means it is no longer available for use as an antioxidant. Total sulfur dioxide, then, is the sum of the free sulfur dioxide (which includes all unbound molecular, bisulfite and sulfite forms) and bound sulfur dioxide (mostly bisulfite bound to its many targets) (Figure 2)1,3,5. Since the proportion of SO2 in any one form in wine is pH dependent, the concentration of molecular SO2 can be calculated using the value for free SO2 and the pH of the wine.
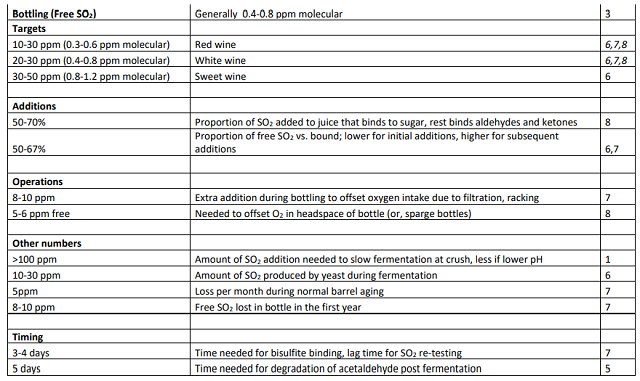
Many guidelines used in winemaking regarding how much SO2 to add to the wine, are based on known quantities needed to inhibit oxidation or microbial growth. Appendix 1 provides a compilation of these quantities from various sources. In many cases, ranges are given rather than firm numbers.
When determining how much SO2 to add, it is important to keep in mind that some of the added SO2 will be bound up by components of the wine. Estimates of SO2 binding are that 1/3 to ½ of the SO2 addition to finished wine will be bound up within the first few days of addition1,2,6. This figure will be higher in younger wines (for which more unbound components exist) as well as wines that have experiences Botrytis infection or infection with acetic acid bacteria, as both are known to produce more compounds that bind SO21,7
The decision of how much SO2 to add to a wine is a good demonstration of the goldilocks principle: one wants to add just enough but not too much. SO2 had many positive effects on sensory perception of wine. Judicious use can:
- preserve the freshness and fruit character of the wine by protecting it from oxidation8
- reverse the nutty, oxidative character of wines caused by acetaldehyde8
- increase extraction of color in red wines9,10
- prevent browning of white and red wines during aging3,9,10
- help prevent microbial spoilage that leads to ethyl acetate and acetic acid8
- prevent malolactic fermentation in crisp white wines8
However, SO2 also has negative sensory effects including
- loss of color due to anthocyanin bleaching8,10
- reduced rate of tannin polymerization and thus maturation in red wines9
- can neutralize other odors of the wine1
- can have negative odors itself such as a metallic, harsh pungent aroma5, wet wool or burning characteristic1
Winemakers are often looking for ways to limit the use of SO2. The best, least risky, way to limit SO2 is to limit the bound fraction of SO2 and maximize the unbound fraction, so that each SO2 addition is more effective. When SO2 is added to wine, it binds to several components including acetaldehyde, thiamine, enzymes, phenolics, and sugars3,11. In white wines, 80% of the bound SO2 is bound to acetaldehyde11 whereas in red wines, most bound to either acetaldehyde or anthocyanins5. In sweet wines, glucose also binds SO2. No matter the wine, reducing acetaldehyde will reduce the bound fraction of SO2 and help limit total SO2.
Acetaldehyde is formed by yeast as an intermediate in alcohol production during fermentation. Ethanol in wine can also be oxidized to acetaldehyde during aging 1,11. It has sensory properties of its own, with a nutty, oxidized aroma sometimes compared to bruised apple8,11. Bisulfite binding converts acetaldehyde to a heavier , non-volatile compound with no sensory impact5. Bisulfite binding to acetaldehyde is relatively strong and rarely reverses, scavenging most SO2 in solution. So ,if there is free SO2, then the acetaldehyde is entirely bound up, packing the bound sulfur fraction1,3,5.
There are three opportunities to control acetaldehyde production: at crush, at the end of fermentation, and during long cellar aging. Saccharomyces cerevisciae produces more acetaldehyde than non-Saccharomyces yeast, with a fairly uniform production rate among strains. The most important variable in acetaldehyde production is the addition of SO2 at crush. SO2 is toxic to Saccharomyces as well as other microbes, so when it is added, they take action to detoxify it. This includes cellular machinery to pump SO2 out of the cell12 as well as enhanced acetaldehyde production. Yeast produce more acetaldehyde as a means of detoxification; SO2 will bind to acetaldehyde before it has a chance to enter their cells and shut down cellular function1,11. So, one way to limit acetaldehyde production is to limit SO2 addition at crush.
Acetaldehyde production by yeast peaks during early fermentation after which time the balance shifts and yeast begin to consume it in the production of ethanol11. A strong fermentation with adequate nutrition produces a larger the number of viable cells at the end of fermentation, which are more likely to consume any remaining acetaldehyde. Other factors that affect acetaldehyde consumption include warmer temperatures (less acetaldehyde is produced) and contact with lees after completion of fermentation11. Malolactic bacteria also consume acetaldehyde simultaneously with and after completion of malolactic fermentation11. As a side note, malic acid bacteria also consume other compounds that bind SO2 including pyruvic acid and alpha keto glutarate11, adding another argument to allowing full malolactic fermentation to reduce SO2 use. Waiting 1-2 weeks after the completion of malolactic fermentation allows time for the consumption of acetaldehyde (as well as diacetyl if it is present) by malic acid bacteria8.
Zoecklein5 states that most acetaldehyde results from microbial oxidation of ethanol under aerobic conditions. Acetaldehyde continues to form during aging as long as oxygen is present. Oxygen can be introduced through any number of means including un-topped tanks, racking and barrel storage. Switching out fermentation bungs for solid bungs, topping regularly, gassing with inert gas, and limiting racking can all diminish acetaldehyde formation during aging. Generally, SO2 can’t compensate for poor cellar practices6.
SO2 addition and effects on tannin evolution
In addition to its potential effects on preserving fruit flavors and aromas, preventing oxidation and spoilage, and potentially muting flavors, SO2 has other impacts on the sensory perception of red wines through its interactions with phenolic groups. In red wines, the main binders of bisulfite are acetaldehyde and anthocyanins. When bisulfite binds to anthocyanins, it turns these pigments colorless, decreasing color intensity2,7. Bisulfite also binds in the same location on the anthocyanin molecule that tannins would bind, therefore SO2 may delay or diminish formation more stable polymeric pigments2. Polymeric pigments that have already formed resist bleaching by SO2 for this same reason2. Binding of SO2 to anthocyanins is reversible, so over time, and with less SO2 present, anthocyanins may be released and gain color1,8, however at wine pH, the bound form is highly favored7.
Another effect of SO2 on polymerization of anthocyanins and tannins is its interaction with acetaldehyde. Acetaldehyde is an important component of tannin polymerization, as it forms bridges between tannin molecules as they form more stable, long-term bonds with each other and with anthocyanins. The presence of SO2 slows the formation of these bonds because it binds with acetaldehyde, essentially taking it out of the equation. Without acetaldehyde, polymerization reactions are slower, including those among catechin molecules (formation of tannins) as well as between catechin and anthocyanins (formation of polymeric pigments)10.
Polymerization of tannins is thought to diminish the astringency and bitterness of wine because larger tannins have less interaction with salivary proteins. Therefore, one would predict an increase in astringency and bitterness with sulfur dioxide use as a result of blocking tannin polymerization. Recent research by Panagiotis et al (2018)13 has added a new wrinkle this prediction. This group has been documenting the presence of sulfonated versions of common sulfur dioxide targets including procyanidins (tannins) and epicatechin (a monomer of tannins). Sulfonation is simply the addition of a sulfonic acid group to an organic compound, in this case, to tannins, catechin and epicatechin, as one would expect to happen when wine is aged in the presence of SO2. Previous studies by the same group had found sulfonated flavenols in red wines (like procyanidins and epicatechin) were positively correlated with age and storage conditions. Here, they surveyed wines of increasing age and found that as wines age in the bottle, these components become increasingly sulfonated.
In a talk at the 69th ASEV conference, Andrew Waterhouse also touched on this idea. He described discovering examples of sulfonated flavonoids (which include anthocyanins and the monomers that make up tannins) during an experiment examining quinone reactions during the phenolic cascade. In these experiments, the flavonoid was sulfonated at the position where tannins would normally bind. Normally, the interflavone bond that holds tannins together is broken by acid hydrolysis during aging. When this happens, the monomers that are released re-polymerize over time to form less astringent tannins. Waterhouse hypothesized that when this happens in the presence of SO2, the SO2 is binding to the subunits instead of another flavonoid monomer, essentially stopping the polymer from growing. He also described finding tannins modified with sulfonation, meaning the chain of monomers was capped by a sulfur group.
One would hypothesize that shorter tannin chains would be more astringent. However, they found that when tannin, acid and SO2 were all present, protein precipitation products were abolished, indicating a softening of the astringency of wines with these conditions. These model wines also saw more SO2 consumed than there was oxygen present, indicating SO2 was binding something else, catechin and epigallocatechin (monomers) after acid hydrolysis.
Taken together, these studies reveal a new element of tannin evolution during aging that may significantly affect the sensory perception of astringency with aging. After discussing the current view of tannin softening by tannin polymerization during aging, Panagiotis et al (2018)13 conclude their work by saying “alongside these reactions, we should now add sulfonated monomeric and dimeric flavenols, which are expected to direct interaction with proteins… sulfonated flavanols, a class of compounds so far neglected, could play a role in improving the sensorial quality of red wine with aging.”
Despite all that is known about the chemistry and interactions of SO2 in wine, many practical questions remain for winemakers. In the following experiment, a single lot of Cabernet Franc wine was divided into six treatments with varying barrel age, dose and timing of SO2 addition. The free and total SO2, SO2 addition rates, volatile acidity and microbiological evolution are reported. Three questions were asked:
- Is it better to add one large addition of SO2 at the end of fermentation and allow the SO2 to drift down during aging, or should many smaller additions be made? The magnitude of the initial addition may have consequences for the microbial population, sensory perception (both preserving fruit flavors as well as potentially producing off aromas), and tannin evolution.
- Is there a difference in managing SO2 in new barrels vs. neutral barrels? Here, do the additional potential binding sites for SO2 on new wood make a noticeable difference in free SO2, and do these have other consequences?
- Can the total sulfur be altered if there is a 2 week lag period between the completion of malolactic fermentation and the addition of SO2? Which is more important, the decrease in acetaldehyde or the risk of volatile acidity accumulation?
Methods
Cabernet Franc grapes were picked on September 26 from a single vineyard block and chilled overnight. On September 27, grapes were sorted, destemmed and loaded into Tbins for fermentation. SO2 (50 ppm) was added to each bin. Bins were inoculated at a rate of 15g/hL of EC1118 yeast the next day (September 28). There was no acid addition. A total of 19 g/L of sugar was added on October 2. Each bin was monitored for Brix and temperature each day. All T-Bins were pressed together on October 10 after the completion of alcoholic fermentation to yield a single lot of wine. Wine was settled in tank for 7 days prior to racking with the addition of 1 g/225L barrel Scott Labs MBR process and 1.1 g/L tartaric acid. Malolactic fermentation was completed in tank. Malic acid depletion was checked every 4 days. Malic acid was determined to be 0.08 g/L on October 26. Wine was racked off lees on October 29, 1 g/L tartaric acid was added in tank, then wine was racked to individual barrels and SO2 was added according to the appropriate treatment regime.
There were several comparisons made in this experiment. The difference in initial dose of SO2 was determined by comparing a “high” dose of 75 ppm SO2 with a “low” dose of 30ppm SO2. For each high vs low comparison, there were three barrels: a new barrel (from Tonnellier Soud Oest with the same forest and toast levels), a neutral barrel (also from the same year, cooper and toast), and a neutral barrel with a delayed dose. The two “normally timed” barrels received SO2 addition 3 days after the end of malolactic fermentation while the “delayed” barrel received SO2 addition 19 days later. There were 6 barrels total (Table 1). Each barrel was periodically monitored for free and total SO2. If needed, SO2 addition was made to maintain a target of 0.5 molecular SO2 (28 ppm).
Sensory Analysis
Sensory analysis was completed in two separate sensory sessions, the first at Bluestone Vineyards in the Shenandoah Valley and the second at Barboursville Vineyards in Central Virginia. At Bluestone, each flight was evaluated by a panel of 12 wine producers. In each flight, wines were presented blind in randomly numbered glasses. There were three tasting groups per flight with balanced tasting order among groups. In the first three flights, wine producers were presented with three wines, two of one type and one of another, and asked to identify which wine was different (a triangle test). Tasters were then asked to score each wine on a scale of 0 to 10 for color, aromatic intensity, fruit intensity and astringency. They were also given open ended questions to describe the wines. Results for the triangle test were analyzed using a one-tailed Z test. Descriptive scores were analyzed using repeated measures ANOVA. These flights included comparisons of (1) high dose vs. low dose wines from neutral barrels (2) high dose vs. low dose wines from new barrels and (3) high dose SO2 addition at 3 days vs. 19 days. A fourth flight presented four wines in randomly numbered glasses: high and low dose SO2 at early and late timing. The wines from new barrels were not included in this flight. Producers were asked to rank the wines in order of preference, with open ended questions available to describe the reason behind the preference. At Barboursville, each flight was evaluated by a panel of 23 wine producers. Three of the four flights were re-tasted, with the sole exception the flight comparing sensory effects of SO2 dose in new barrels.
Results
Initial juice chemistry was measured after destemming. Juice had 19.2°Brix with a pH of 3.85. After chaptalization and acidulation, the wine finished malolactic fermentation with pH of 3.54 and 11.9% alcohol.
Overall SO2 addition and Total SO2
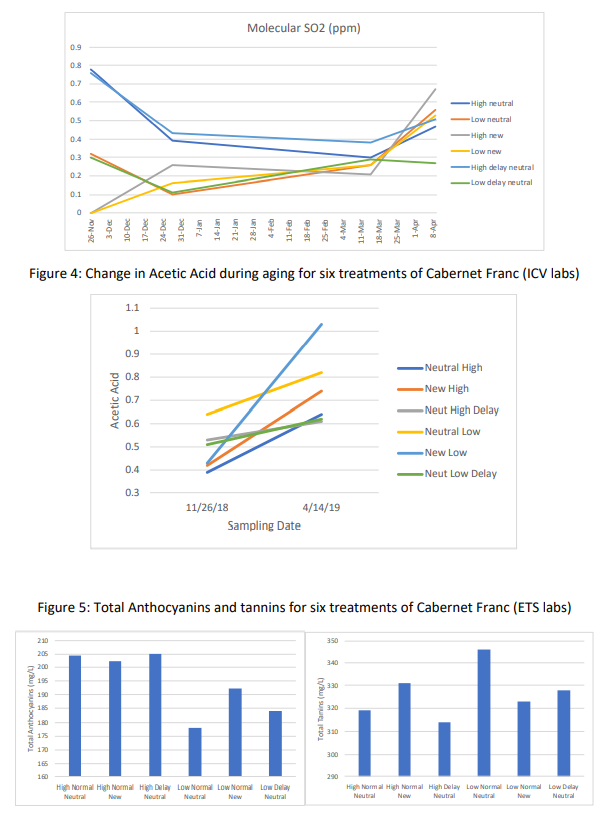
A higher initial rate of SO2 addition led to higher total SO2 in the finished wine. In all cases, the high rate addition had higher total sulfur than its low dose counterpart (Table 2). The barrel receiving a delayed high dose required notably lower SO2 addition during aging (Table 2) while maintaining a higher level of molecular SO2 than the other treatments for the majority of the time of aging (Figure 3). This wine also had a lower total SO2 at the end of aging than the other wines with an initial high dose (Table 2). Despite routine monitoring and addition, none of the barrels were able to maintain the target molecular sulfur throughout aging. The high dose barrels were closer to the target while the low dose barrels were considerably below this target at nearly every sampling event (Figure 1).
Differences in wine chemistry
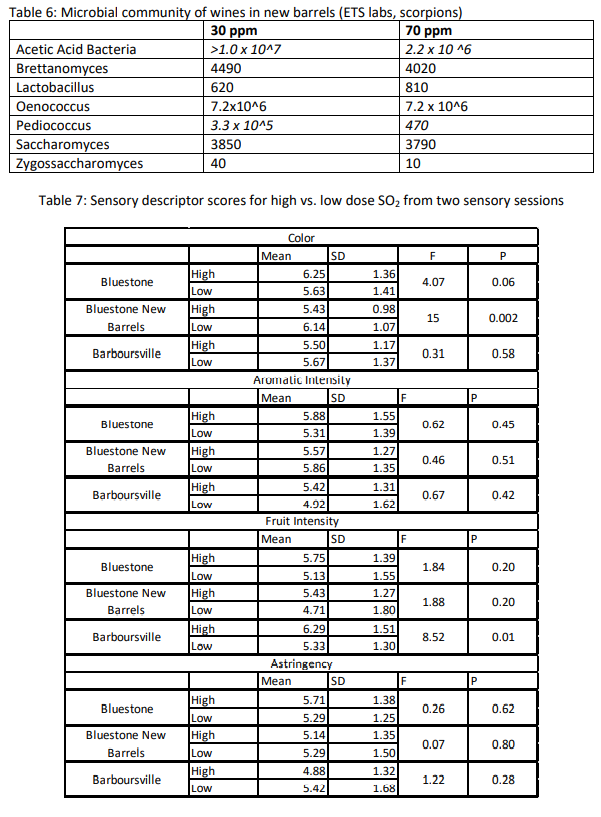
Four of the six barrels maintained acetic acid accumulation rates within the expected range of 0.05 g/L per month (Table 3, Figure 4). The new barrels showed higher acetic acid accumulation rates than their counterparts, likely due to higher rates of SO2 binding to oak components, leaving less free SO2. The low dose new barrel had unacceptably high accumulation of acetic acid, likely due to infection with acetic acid bacteria. Though both delayed dose barrels had higher initial acetic acid than their counterparts, both maintained a lower overall level of acetic acid accumulation, leading to the lowest acetic acid levels at the end of the aging period (Table 3, Figure 4).
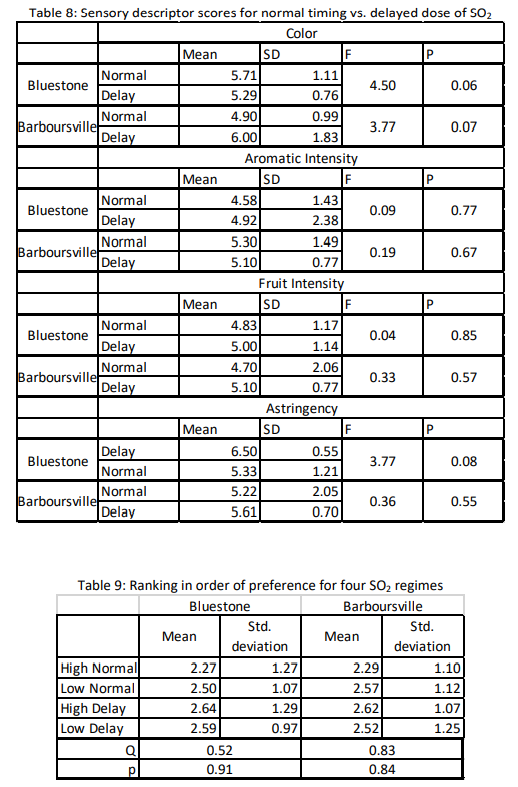
With the exception of the high dose barrel, all other barrels lost color through the course of aging (Table 4). Though the delayed dose barrels had a higher amount of color loss, they also started with higher color, leading to average color levels at the end of the aging period. There were not consistent trends in color loss based on initial SO2 dose or timing. However, anthocyanins were always higher in the higher dose wines and lower in the lower dose wines (Table 5, Figure 5). Tannins tended to be slightly higher in low dose wines, with the exception of the new barrels. Tannins were generally higher in the new barrels (Table 5, Figure 3).
The new barrel receiving a low dose of SO2 had much higher accumulation of acetic acid than the other three barrels. Sensory analysis indicated microbial spoilage, so microbiological testing using a PCR panel was done to describe the microbiological community of the wines in the new barrels (Table 6). The low dose barrel had much higher populations of acetic acid bacteria and Pediococcus. Both of these groups are relatively resistant to SO2, so it is likely that the low dose was not sufficient to inhibit activities while the higher dose of SO2 had a greater effect.
Results of sensory analysis
In a triangle test of high vs. low SO2 dose wines, 8 out of 12 respondents were able to distinguish which wine was different in the Bluestone tasting and 12 out of 23 respondents were able to distinguish which wine was different at the Barboursville tasting. In both cases, the wines were significantly different (Z=2.14 and 1.7, p=0.02 and 0.04, respectively). This difference was also seen in the comparison of doses in wines aged in new barrels (Z=1.81, p=0.04). However, descriptions for what made the wines different in open ended questions, as well as scores for specific sensory descriptors (Table 7) did not show consistent results. For example, color was perceived to be higher in the high dose barrels at Bluestone but only in the neutral barrels. The new barrels showed the opposite trend.
In a triangle test comparing high dose SO2 addition shortly after completion of malolactic fermentation with addition after two weeks, 7 out of 12 respondents were able to distinguish which wine was different in the Bluestone tasting and 10 out of 23 respondents were able to distinguish which wine was different at the Barboursville tasting. The difference was significant at the Shenandoah tasting (Z=1.81, p=0.035) but not at the Barboursville tasting (Z=0.81, P=0.21). Once again, trends were not consistent between the two tastings. For example, color was scored higher in the normally timed barrels at Bluestone but higher in the barrels with delayed timing at Barboursville. It is also important to note there are only small differences in means among any of the descriptors. When asked to rank the wines in order of preference, none of the wines was significantly preferred over any of the others (Table 9).
Conclusions
- Is it better to add one large addition of SO2 at the end of fermentation and allow the SO2 to drift down during aging, or should many smaller additions be made? High initial dose led to higher total sulfur in each case. However, high initial dose was also closer to the target SO2 rate for aging for a higher proportion of the aging period. High initial dose may have helped preserve anthocyanins and had lower overall accumulation of acetic acid during aging.
- Is there a difference in managing SO2 in new barrels vs. neutral barrels? New barrels had lower free SO2 than their neutral counterparts on the same SO2 schedule. They accumulated higher amounts of acetic acid and had high microbial populations of Pediococcus and acetic acid bacteria. If using new barrels, care should be taken to monitor SO2 levels early in aging to accommodate for additional SO2 binding by new barrels vs. older barrels.
- Can the total sulfur be altered if there is a 2 week lag period between the completion of malolactic fermentation and the addition of SO2? Delaying SO2 addition by two weeks decreased total sulfur levels at both dose levels. These wines began with higher acetic acid but experienced very little acetic acid accumulation during aging. The high, delayed dose was closest to the target SO2 during aging.
- Though these wines were distinguishable from one another during sensory analysis, there was no significant preference for one wine over the other and no consistent trends in the differences (color, astringency, fruit or aromatic intensity).
References
(1) Ribereau-Gayon, P.; Dubourdieu, D.; Doneche, B.; Lonvaud, A. Handbook of Enology Volume 1: The Microbiology of Wine and Vinifications, 2nd ed.; John Wiley & Sons: West Sussex, England, 2006.
(2) Zoecklein, D. B. Sulfur Dioxide (SO2). Enology Notes Downloads, 16.
(3) Boulton, R.; Singleton, V. L.; Bisson, L. F.; Kunkee, R. E. Principles and Practices in Winemaking; Chapman and Hall, Inc: New York, 1996.
(4) Rotter, B. Sulfur Dioxide. Improved Winemaking: advanced theory, practical solutions and opinions.
(5) Zoecklein, B. W. Sulfur Dioxide: Science behind This Antimicrobial, Antioxidant, Wine Additive. Practical Winery and Vineyard Journal 2009.
(6) Stamp, C. Methods for Calculating SO2. Wines and Vines 2011.
(7) Margalit, Y. Concepts in Wine Chemistry, 3rd ed.; The Wine Appreciation Guild LTD: San Francisco, California, 2012.
(8) Stamp, C. How Much SO2 to Add and When. Wines and Vines 2011.
(9) Gomez-Plaza, E.; Gil, R.; Lopez-Roca, J.; Adrian, M. Effects of the Time of SO2 Addition on Phenolic Compounds in Wine. Vitis -Geilweilerhof- 2001, 40, 47–48.
(10) Picinelli, A.; Bakker, J.; Bridle, P. V. Model Wine Solutions: Effect of Sulphur Dioxide on Colour and Composition during Ageing; 2015.
(11) Jackowetz, N.; Li, E.; de Orduña, R. M. Sulphur Dioxide Content of Wines: The Role of Winemaking and Carbonyl Compounds. Appellation Cornell 2011, 3, 1–7.
(12) Bisson, L. F. Geographic Origin and Diversity of Wine Strains of Saccharomyces. American Journal of Enology and Viticulture 2012, 63 (2), 165–176. https://doi.org/10.5344/ajev.2012.11083.
(13) Arapitsas, P.; Guella, G.; Mattivi, F. The Impact of SO2 on Wine Flavanols and Indoles in Relation to Wine Style and Age. Scientific Reports 2018, 8. https://doi.org/10.1038/s41598-018-19185-5.
|
Appendix 1 References |
|
|
1. Bokulich, Nicholas A., Michael Swadener, Koichi Sakamoto, David A. Mills, and Linda F. Bisson. “Sulfur Dioxide Treatment Alters Wine Microbial Diversity and Fermentation Progression in a Dose-Dependent Fashion.” American Journal of Enology and Viticulture 66, no. 1 (February 1, 2015): 73–79. https://doi.org/10.5344/ajev.2014.14096. |
|
|
2. Boulton, Roger, Vernon L. Singleton, Linda F Bisson, and R.E. Kunkee. Principles and Practices in Winemaking. New York: Chapman and Hall, Inc, 1996. |
|
|
3. Cojocaru, George, and Oana Antoce. “Chemical and Biochemical Mechanisms of Preservatives Used in Wine: A Review.” Sci. Pap. Ser. B: Hortic. 56 (November 24, 2015): 457–66. |
|
|
4 Jackowetz, Nick, Erhu Li, and Ramón Mira de Orduña. “Sulphur Dioxide Content of Wines: The Role of Winemaking and Carbonyl Compounds.” Appellation Cornell 3 (2011): 1–7. |
|
|
5. Margalit, Yair. Concepts in Wine Chemistry. 3rd ed. San Francisco, California: The Wine Appreciation Guild LTD, 2012. |
|
|
6. Ribereau-Gayon, Pascal, Denis Dubourdieu, Bernard Doneche, and Aline Lonvaud. Handbook of Enology Volume 1: The Microbiology of Wine and Vinifications. 2nd ed. West Sussex, England: John Wiley & Sons, 2006. |
|
|
7. Stamp, Chris. “How Much SO2 to Add and When.” Wines and Vines, May 2011. |
|
|
8. Zoecklein, Dr Bruce. “Sulfur Dioxide (SO2).” Enology Notes Downloads (n.d.): 16. |
