SO2 Chemistry in a Nutshell
Joy Ting
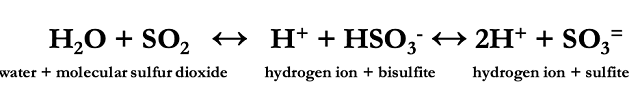
When used in the winery, SO2 is most often in a liquid form. When SO2 dissolves in water, it doesn’t remain only as SO2. Instead, it interacts with the water in a way that takes three forms: molecular sulfur dioxide, bisulfite and sulfite (see equation). The amount of any one form that is present depends on the pH of the solution, with the equation shifted to the left in low pH environments and to the right in high pH environments. This means that, the higher the pH, the less molecular sulfur dioxide is available (1–3). These relationships are also in dynamic equilibrium, so if SO2 is removed from solution, say by entering into a microbial cell, sulfite ions will give up another hydrogen ion to replenish the “missing” molecular SO2. Likewise, if a bisulfate anion binds to an acetaldehyde, a molecular SO2 molecule will gain and O and an H to replace it. This happens only as much as the remaining ions are available such that if the balance is 20%, 60%, 20%, they will always be in this balance, regardless of what concentration that means for each constituent. (At wine pH, this is more realistically 2%, 98%, 0.1%). Also, if SO2 is added to the solution, even if it is added in the form of molecular SO2, it will quickly redistribute to its various forms based on pH (1–3).
The figure below gives a graphical representation of the relationship between pH and the forms of sulfur dioxide. Between pH of 3 and 4, which describes most wine, bisulfite is the predominant form of sulfur dioxide while molecular sulfur and sulfite are scarce. At a pH of 3.0, molecular sulfur dioxide makes up 5.6% percent of the SO2, bisulfite makes up 94.4% and the sulfite ion is makes up only 0.006%. At pH of 4, these numbers are 0.585%, 99.4%, and 0.06%, respectively (3). This balance of forms is important because not all forms of sulfur dioxide have the same activity.
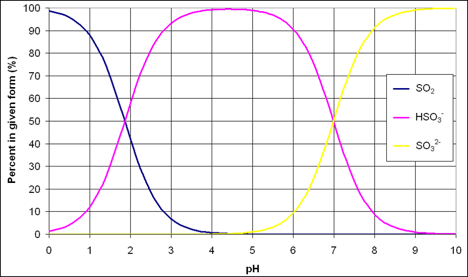
from Rotter n.d. (4)
References
(1) Ribereau-Gayon, P.; Dubourdieu, D.; Doneche, B.; Lonvaud, A. Handbook of Enology Volume 1: The Microbiology of Wine and Vinifications, 2nd ed.; John Wiley & Sons: West Sussex, England, 2006.
(2) Zoecklein, D. B. Sulfur Dioxide (SO2). Enology Notes Downloads, 16.
(3) Boulton, R.; Singleton, V. L.; Bisson, L. F.; Kunkee, R. E. Principles and Practices in Winemaking; Chapman and Hall, Inc: New York, 1996.
(4) Rotter, B. Sulfur Dioxide. Improved Winemaking: advanced theory, practical solutions and opinions.
