Effect of Micro-oxygenation in Merlot
Michael Heny
Michael Shaps Wineworks
Summary
Micro-oxygenation is a controlled use of oxygen. Skillful application of this tool can lead to better color, structure, and sensory properties in red wines, but any addition of oxygen also comes with risks. In this experiment, a single lot of Merlot with notable astringency was split into two lots post-malolactic fermentation. One lot received no additional oxygen beyond normal cellar operations (racking and barrel aging). The other lot was treated with 5 mL/L/month for 10 hours every other day for 14 days. After SO2 addition and several months of aging, there was no notable difference in basic wine chemistry. The micro-oxygenated wine had lower measured values for monomeric and total anthocyanins, and higher color hue. Sensory analysis was not conducted due to COVID-19 restrictions.
Introduction
Winemakers have a complicated relationship with oxygen. At times, they fiercely protect juice and wine from its presence, excluding it in any way possible. At other times, they invite it in and hope it does its work. It all depends on the wine, and the situation. Micro-oxygenation is a controlled use of oxygen. Skillful application of this tool can lead to better color, structure, and sensory properties in red wines, but any addition of oxygen also comes with risks.
There are several steps during the winemaking process when oxygen is introduced into juice and wine. Oxygen is introduced almost every time the wine is moved and even during storage (Table 1). After introduction to the wine, oxygen is quickly bound by various components, such as ethanol (to form acetaldehyde), phenolics (tannins and anthocyanins), SO2, aroma and flavor compounds, which can have a profound effect on astringency and aroma.
Table 1: Oxygen introduction during winemaking operations1,2
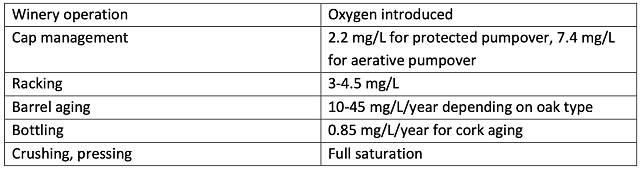
Unlike the uncontrolled addition of oxygen during cellar operations, micro-oxygenation (MOX) is the process of intentionally adding small amounts of oxygen in a measured way to induce favorable changes in color, aroma, and texture3. The technique was first developed in France in the 1990’s as a way of aging tannic red wines in stainless steel tanks rather than oak barrels. It is now also used to accelerate aging, stabilize color, and treat reduction and herbaceous/green character. Rather than shortening the life of a wine, as oxidation normally does, MOX can increase the anti-oxidative power and potential aging of the wine by stimulating phenolic reactivity1.
When oxygen is introduced to wine, it diffuses randomly and encounters many chemical compounds in the wine. Oxygen is generally highly reactive and will show the most effects in those chemicals that are found in the highest concentration in wine, such as ethanol, anthocyanins, tannin subunits, and tannins themselves. Oxygen will also interact with molecules that are responsible for aroma and flavor. Though a winemaker may be targeting one component, oxygen will interact with them all.
One of the most common reasons to micro-oxygenate wine is to help structure tannins. Phenolics such as anthocyanins and tannins are responsible for the color and mouthfeel (astringency) of red wine. Micro-oxygenation can alter the polymerization rate and bonding arrangement of tannins and pigments, leading to less astringency and more color stability. Micro-oxygenation can also reduce the perception of reduced compounds such as H2S (sewer gas, rotten eggs) and methyl mercaptan (cabbage, skunk)1,4.
When it was first introduced, reports showed that micro-oxygenation also led to significant decreases in the sensory perception of herbal/vegetal character in wine. However, repeated measurement of the chemical methoxypyrazine, a principle chemical component of “veggie”, did not change. Further study revealed that the perception of “veggie” included a combination of pyrazine as well as some reduced sulfur compounds (AKA thiols) with vegetal aromas (such as canned green beans). The reduction of the perception of “veggie” after micro-oxygenation was due to oxidation of these reduced compounds4,5.
Along with its potential benefits, micro-oxygenation also has some risks. Allowing micro-oxygenation to go on too long can lead to excessive polymerization, making tannins dry and harsh4. Oxygenation at too high of a rate can leave excess oxygen in the wine that reacts with anthocyanins to cause browning and oxidation of varietal aromas and flavors6. Oxygen availability in wine can also lead to microbial spoilage. Acetobacter will use available oxygen to produce acetic acid. Brettanomyces growth is facilitated by the presence of oxygen4(though it is thought that at low levels, micro-oxygenation poses less of a threat than standard racking3). Zoecklein 1 likens the management of micro-oxygenation to piloting an ocean liner. It must be done carefully and pro-actively with the understanding that one action begets a chain reaction that is not easy to control or fully predict. A firm understanding of the underlying mechanisms at play and careful monitoring of progress are key to success.
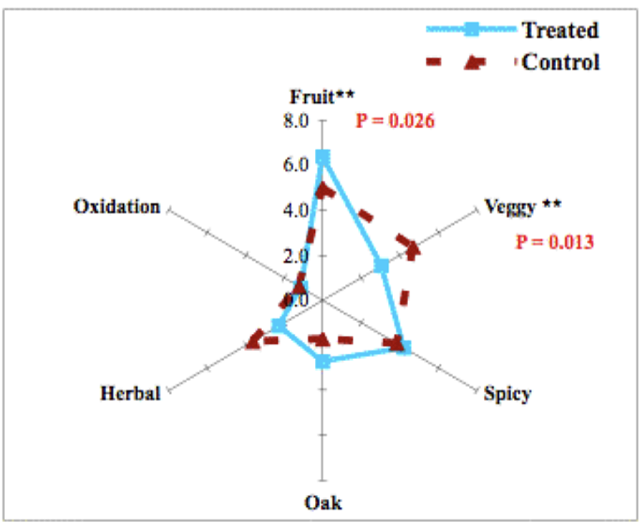
From: Enology Notes #134, Zoecklein (2007)5
To micro-oxygenate wine, an oxygen supply (purchased from the gas supplier) is connected to a dosing chamber (such as the Stavin Ox Box). The dosing chamber acts as a high precision dosing regulator that can be set for very small amounts of oxygen flow. Oxygen exits the box through small tubing into miniature sparging tips with very small (10 um) pore size to produce very fine bubbles7. Soluble solids and lees have been shown to interfere with MOX effects8, and can contain spoilage microbes, so the tubing is configured so that the sparging tips are suspected about an inch above the bottom of the tank to avoid disturbing any remaining lees.
Figure 1: Schematic representation of a micro-oxygenation system. From Gomez-Plaza and Cano-Lopez (2010)3
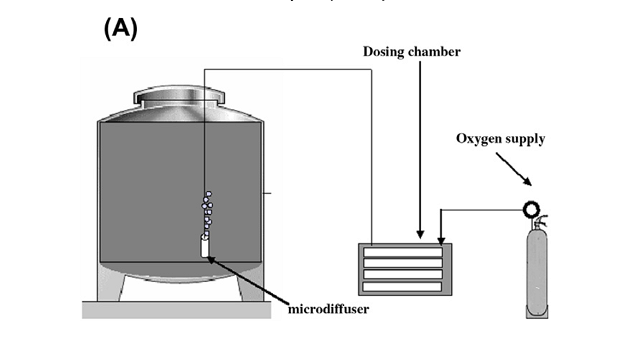
MOX can be applied at any time during vinification and maturation, with total doses up to 600 mL/L for tannic red wines over the lifetime of the wine8. The effects of MOX will differ depending on the timing and dose rate of the addition. MOX has been used during fermentation as a means to encourage yeast health and reduce off aromas during fermentation. MOX prior to malolactic fermentation (MLF) has been shown to be effective in improving wine structure8. After malolactic fermentation, it is thought that at least some polymerization has already occurred, so MOX has less of an effect on tannin structure. However, due to the high number of wines needing attention during harvest, applying MOX after MLF may be more practical. It is not uncommon for MOX to be done at higher rates before malolactic fermentation, stopped while malic acid bacteria are active, then applied again at lower rates after malic acid has been depleted3,8.
In this experiment, Merlot with high astringency was treated with micro-oxygenation to soften the rough tannins. Micro-oxygenation was performed after malolactic fermentation was complete. Chemical properties were assessed after aging.
Methods
Merlot underwent alcoholic and malolactic fermentation as a single lot prior to splitting for experimentation. This lot was produced according to the standard procedures of the winery. Grapes were hand harvested, refrigerated overnight, then destemmed and transferred through a must pump to a temperature controlled stainless steel fermentation tank and several TBins with the addition of SO2, 0.1 mL/L Color Pro, 2.5 g/L Nadalie FMT+ chips, and 0.2 g/L Tannin VR Supra. Must was inoculated the same day with 0.2 g/L D254 yeast. Fermentations were pumped over twice per day with daily fermentation monitoring. Nutrients (Superfood and DAP) were added in two parts, on days 2 and 3 of fermentation. Tartaric acid (0.5 g/L) was also added on day 3. Wine was pressed after 31 days of total maceration.
After pressing, wine was allowed to settle for three days prior to racking off settled lees to a clean tank, then the lot was split into separate vessels for control and treatment. One portion was racked to a neutral French oak barrel (control) while the other portion was racked to a 1200 L cylindrical stainless steel tank with at least 4 feet of vertical distance for micro-oxygenation. Turbidity was measured after racking with a target of less than 200 to prevent aerobic spoilage organisms and particulate binding sites for oxygen. A lead line from the Stavin Ox Box was threaded into the treatment tank so that the dosing stone was suspended 6 inches from the bottom of the tank to avoid lees. The line remained in place for the duration of the experiment to avoid oxygen addition from disturbing the surface.
Due to the small volume of the treatment tank (1125 L), continuous dosing was not possible from the Ox Box. Instead, a discontinuous rate was set targeting 5 mL/L/month, at a dose rate of 0.61 mL/min for 10 hours roughly every other day. Temperature was checked prior to each oxygen dose. Throughout the experiment, the treatment lot was monitored weekly for sensory attributes (specifically tannin structure, offensive odors, and oxidation). Treatment was halted according to the discretion of the winemaker based on this assessment, in this case after two weeks. Wine was transferred from the treatment tank to barrels after one month. After one additional month, 0.25 g/L tartaric acid and 50 ppm sulfur dioxide was added to both lots. SO2 was checked monthly and maintained to a common target.
Results
Fruit chemistry (Table 2) and wine chemistry after pressing (Table 3) are within norms for Virginia Tannat. The wine completed malolactic fermentation during extended maceration in tank, prior to pressing and separation of lots. Post-treatment wine chemistry is very similar between treatment lots (Table 4).
One concern with micro-oxygenation is the potential accumulation of acetaldehyde that can lead to additional binding of SO2. Both lots received 50 ppm SO2 on 12/20 (after completion of MOX treatment). In-house measurement of free SO2 on 1/31 indicated the control had 24 ppm fSO2 leading to an addition of 10 ppm while the MOX treatment had 19 ppm fSO2, leading to an addition of 20 ppm. Subsequent measurements led to comparable additions. The additional SO2 needed for the MOX treatment may be due to slight accumulation of acetaldehyde during oxygenation, however, this did not result in higher overall total SO2 (Table 4).
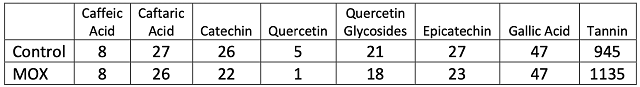
There was little difference in color intensity for the two wines (Figure 2), however, there was a shift in hue from 0.72 in the control to 0.86 in the micro-oxygenated wine. This indicates a shift from the red to the yellow end of the spectrum, a shift also seen in aged wines. Wine color is affected by many factors such as SO2 and pH, so it is difficult to draw conclusions from color differences. The wine that received micro-oxygenation also showed a decrease in anthocyanins (Table 5). Notably, loss of monomeric anthocyanins did not lead to an increase in polymeric anthocyanins, but rather led to overall loss of total anthocyanins. There were very few differences in concentration of phenolics between treatments (Table 6). Micro-oxygenation is not expected to remove phenolics but rather affect the structures of polymers, an effect that would be not seen in these values. At present, the best way to assess changes in phenolic structure is through sensory analysis. Unfortunately, due to public health measures put in place during the COVID-19 pandemic, sensory analysis was not completed prior to blending of these wines.
Table 2: Fruit chemistry for Merlot at harvest (in-house data)

Table 3: Merlot wine chemistry after pressing (in-house data)

Table 4: Merlot wine chemistry after separation and treatment with MOX (ICV labs)
Figure 2: Color intensity for two treatments of Merlot (ICV labs)
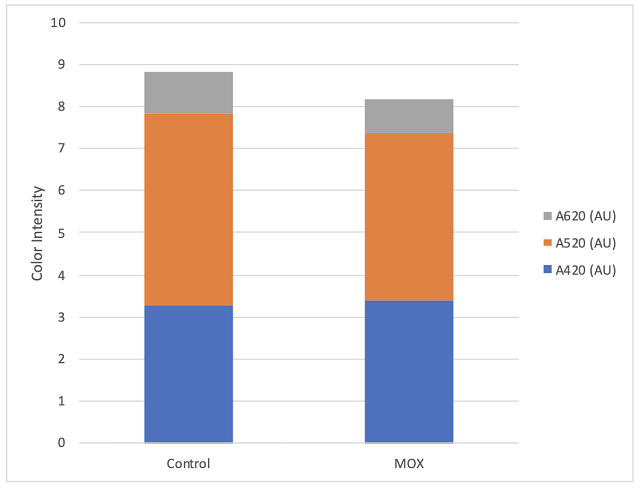
Table 5: Anthocyanins found in two treatments of Merlot (mg/L) (ETS labs)
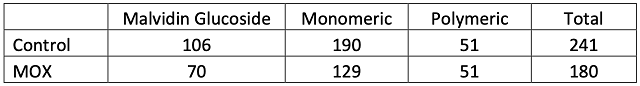
Table 6: Phenolic compounds measured in two treatments of Merlot (mg/L) (ETS labs)
References
(1) Zoecklein, B. W. Winemaking Topics; Micro-Oxygenation. Virginia Tech Wine/Enology Grape Chemistry Group, n.d.
(2) Waterhouse, A. L.; Laurie, V. F. Oxidation of Wine Phenolics: A Critical Evaluation and Hypotheses. American Journal of Enology and Viticulture 2006, 57 (3), 306–313.
(3) Gómez-Plaza, E.; Cano-López, M. A Review on Micro-Oxygenation of Red Wines: Claims, Benefits and the Underlying Chemistry. Food Chemistry 2011, 125 (4), 1131–1140.
(4) Zoecklein, B. W. Current Theory and Applications: Microoxygenation. Practical Winery and Vineyard2007, November/December.
(5) Zoecklein, B. W. Factors Impacting Sulfur-like Odors in Wine and Winery Operations Part 2. Enolgoy Notes #134, 2007.
(6) Singleton, V. L. Oxygen with Phenols and Related Reactions in Musts, Wines, and Model Systems: Observations and Practical Implications. 1987, 38 (1), 69–77.
(7) McCord, J. Micro-Oxygenation: A Treatise. Stavin, 2009.
(8) Lesica, M.; Košmerl, T. Microoxygenation of Red Wines. Acta agricultura Slovenica 2009, 93 (3), 327–336.
