Effect of yeast strain and chitosan addition on fermentation kinetics, protein stability, and sensory properties of Sauvignon Blanc (2020)
Matthieu Finot
King Family Vineyards
Summary
In 2019, King Family Vineyard began producing Bordeaux-style Sauvignon Blanc with barrel fermentation. Experiments in 2019 revealed differences due to yeast strain, as well as prevalence of unwanted malolactic fermentation during aging on lees in a low SO2 environment. In 2020, experiments were conducted to further explore the effects of yeast strain for aromatics and chitosan to prevent malolactic fermentation. Despite the addition of 15 g/hL during fermentation without racking during aging, several barrels underwent partial or full malolactic fermentation. This occurred in wines inoculated with commercial yeast as well as those inoculated using a vineyard starter culture. Wine fermented with the vineyard starter culture with and without chitosan were significantly different in a triangle test, with the chitosan treated wine receiving significantly higher scores for citrus character. A higher dose if Stab Micro M may be needed to prevent malolactic fermentation in a low SO2 environment when wine is aged on lees.
Introduction
In 2019, King Family Vineyard began producing varietal Sauvignon Blanc. Sauvignon Blanc has been shown to take on different sensory descriptors depending on its region of origin1 as well as many aspects of winemaking. One group of molecules that has come to exemplify New Zealand style Sauvignon Blanc are the thiols2. Thiols can be affected by conditions in the vineyard (water stress, nutrient additions, defoliation, fruit damage)3, practices in the winery (yeast group, skin contact, stabulation, specialized nutrients)4–8 and during aging (reductive environment, time)9. There is more information on thiols in general in the Learn Section of the WRE website. When formulating a winemaking plan for Sauvignon Blanc at King Family, Matthieu Finot desired a mineral-driven, Bordeaux style Sauvignon Blanc rather than a high thiol New Zealand style Sauvignon Blanc. With these elements in mind, the winemaking protocol for King Family Sauvignon Blanc was designed with permissive oxygen at the press, barrel fermentation, and did not include elements meant to increase thiols such as dry ice/CO2 blanketing, specialized nutrition or added enzymes. After fermentation, wine was aged in barrels on lees with low free SO2.
The choice of yeast strain for Sauvignon Blanc has a considerable impact on the flavor profile and varietal expression4. In addition to converting sugar to alcohol during fermentation, yeast convert non-volatile precursors to aromatic molecules through the action of enzymes. Enzymes are genetically encoded and yeast strains may have different variants of genes, leading to different ability among strains to convert precursors to volatile compounds2,3,10,11. For example, there can be up to 25-fold difference in the expression of thiols in wines produced by one yeast strain vs another (Bruce Zoecklein, personal communication). Enological companies now market specific yeast strains as “thiol producing”, because they have a high number of precursor-converting genes.
An experiment conducted in 2019 testing the use of two commercial yeast strains and an ambient starter culture in Sauvignon Blanc at King Family showed notable differences in fermentation kinetics, malolactic fermentation and sensory descriptors for these three yeast groups. Wine fermented with Zymaflore X5 showed robust fermentation kinetics, little malic acid conversion, and significantly higher scores for traditional Sauvignon Blanc descriptors such as citrus, boxwood, tropical fruit, green character and complexity when compared to wines fermented with Diana and an ambient starter culture. The wine fermented with the ambient starter culture fermented slower and completed with fermentation with higher volatile acidity and notable depletion of malic acid. Despite a permissive oxygen environment, the X5 yeast still produced a high thiol (New Zealand style) wine. The sensory profile of the ambient starter culture was closer to the desired style found in French Sauvignon Blanc, however these wines finished with higher volatile acidity and some sensory characteristics of malolactic fermentation.
In the current study, three yeast strains (X5, Q9, and a vineyard starter culture) will once again be utilized. In addition, malolactic fermentation will be discouraged by the use of chitosan. Chitosan is a naturally occurring molecule that can also be produced by the de-acetylation of chitin using NaOH or chitinase enzymes12. Chitin is the second-most common polymer found in nature (after cellulose), making up the cell walls of fungi and shells of crustaceans and is thus readily available as a renewable resource13. Chitosan has been used worldwide at nearly every stage of wine production including vineyard applications, on grapes during transport and storage, at crush, after fermentation and during aging of wine14. During fermentation and aging, chitosan binds to the cell walls of gram negative bacteria (such as Oenococcus and Lactobacillus), causing possible disruption of their cell membranes, ATP leakage, and sedimentation15,16. Prevention of malolactic fermentation using chitosan may lead to more robust fermentation, retention of malic acid, and lower volatile acidity in the ambient fermentation of Sauvignon Blanc.
Methods
Approximately 5 days prior to harvest, a vineyard starter culture was prepared according to the standard protocol of King Family (Appendix A). Grapes were hand harvested on 9/4 and chilled overnight prior to processing. Grapes were whole cluster pressed on 9/5 with the addition of 3 mL/ton Lafase XL Press enzyme, 0.12 g/L Stab Micro M (a chitosan product) and 20 ppm SO2. The following day, an additional 20 ppm of SO2 was added along with 43 g/hL
Endozyme Antibotrytis. Juice settled for three days then was racked off settled lees. Clarified juice received 30 g/L sugar and 0.5 g/L tartaric acid, then was transferred to barrels for fermentation. For X5 and Q9 fermentations, 0.2 g/L yeast was rehydrated in 0.25 g/L Go Ferm Protect Evolution. Fermentation was carried out in a cool cellar. Once a robust fermentation had begun (to allow for self-mixing), on 9/18/20, 0.15 g/L Stab Micro M was added to the fermentation in the treatment barrels. The control barrels did not receive Stab Micro M at this stage. Fermentations were monitored daily for specific gravity and temperature. At the completion of fermentation, residual sugar was measured enzymatically. When glucose/fructose was below 1.0 g/L, barrels were treated with 40 ppm SO2. Wine was aged on lees in a low SO2 environment. Additional tartaric acid (0.6 g/L) was added after chemistry was evaluated in December.
Sensory analysis comparing wine made from the vineyard starter culture with and without the use of chitosan was completed by a panel of 17 wine producers. Due to restrictions put in place during COVID-19, sensory analysis was completed using shipped samples. Each wine producer received three wines in identical bottles, filled on the same day, each coded with random numbers. Two of the bottles contained the same wine while the third bottle contained the different wine. Participants were asked to identify which wine was different (a triangle test). There were four tasting groups with the unique wine in the triangle test balanced among the groups. Participants were then asked to score each wine on a scale of 0 to 10 for citrus, boxwood/broom/cat pee, tropical fruit, green character, flint/stone/mineral, and complexity. They were also given open ended questions to describe the wines. Results for the triangle test were analyzed using a one-tailed Z test. Descriptive scores were analyzed using repeated measures ANOVA.
Results
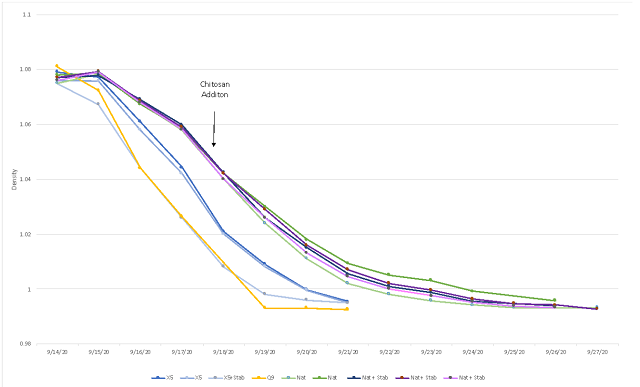
Fruit was harvested in early September with 17°Brix and a pH of 3.55. Additions of sugar and tartaric acid increased the Brix and decreased the pH (Table 1). Of the three yeast groups utilized, Q9 fermented the fastest with the vineyard starter culture fermenting the slowest (Figure 1). However, these were all robust fermentation curves without the sluggish nature of fermentation seen in 2019. All of the treatments completed fermentation with residual sugar less than 1.0 g/L (ICV labs, data not shown). This was true for all fermentations, regardless of whether or not chitosan was added (Figure 1). In 2019 there was little malolactic fermentation in the fermentations inoculated with X5 yeast. In 2020, however, the wines fermented with X5 appear to have undergone malolactic fermentation during aging (Table 2). These wines had noticeable reductive notes, which may be the result of malolactic fermentation. The fermentation inoculated with Q9 aged with nearly 3 g/L malic acid still present in April (Table 2). The wines produced by the ambient starter culture also completed fermentation with much of its malic acid present, however, some malolactic conversion is evident during aging in both the treatments with chitosan and those without (Table 2).
Chitosan treatment has also been suggested for the removal of unstable proteins in wine. Chitinase is one of the most unstable proteins in wine; chitosan is a substrate for chitinase. Studies have shown that large amounts of chitosan addition (1 g/L) can result in protein stable wines, however additions at this rate are not financially practical17. Recent trials at Enartis have shown that aging wine on chitosan with periodic battonage can increase its efficacy as an antimicrobial and protein stabilizing agent (Jasha Karasek, personal communication). Other studies have shown that treatment of Saccharomyces with chitosan causes damage to cell walls that is then repaired by adding higher than usual amounts of chitin, another substrate of chitosan. This leads to the hypothesis that fermentation in the presence of chitosan at reasonable rates (10-30 g/hL) may have the synergistic effect of resuspension as well as selection for higher chitin in yeast cell walls, leading to the potential for protein stable wines. This did not appear to be the case for King Family Sauvignon Blanc (Table 3). Protein stability testing indicated that these wines required between 4-6 pounds/1000 gallons of bentonite to achieve stability. Barrel 1390, which was treated with chitosan, appears to be slightly more stable, but this effect was not replicated in barrel 1691, which also received chitosan treatment.
In a triangle test, 12 out of 17 respondents were able to distinguish which wine was different, indicating the wines were significantly different (Z=3.00, p= 0.0013). The wine treated with chitosan received significantly higher descriptive scores for “citrus” than the wine without chitosan. None of the other descriptive scores were significantly different (Table 4).
The recommended dose of Stab Micro M to be used during fermentation is 10-30 g/hL. In this experiment, 15 g/hL was used. Free SO2 was <7ppm in each barrel at each testing event. A higher dose if Stab Micro M may be needed to prevent malolactic fermentation in a low SO2 environment when wine is aged on lees.
Table 1: Juice analysis before and after additions of sugar and acid (in-house data)
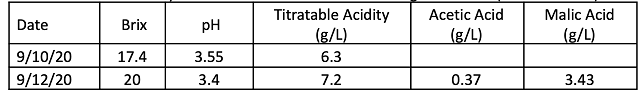
Table 2: Finished wine chemistry from two time periods during aging (ICV Labs)
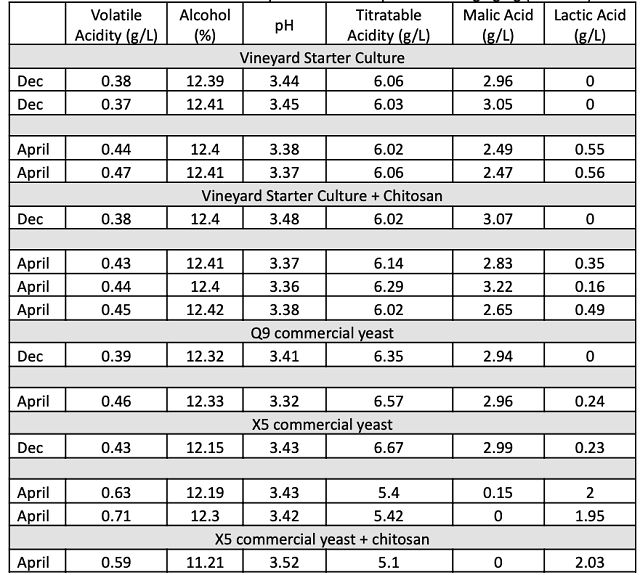
Table 3: Protein instability post fermentation for several yeast groups (ETS Labs)
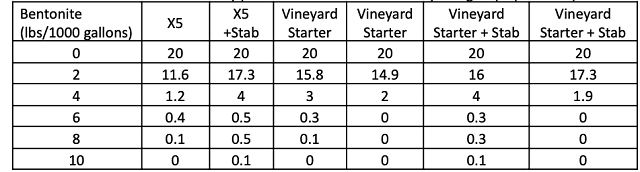
Table 4: Statistical analysis for descriptive scores from blind sensory analysis Sauvignon Blanc
Figure 1: Fermentation kinetics for three yeast groups and treatments of Sauvignon Blanc (In-house data)
Appendix A: Vineyard starter culture protocol
To prepare a starter for the ambient fermentation, 4-5 days prior to harvest, collect clean clusters and crush them into a cleaned and sanitized 6 gallon bucket with a removable lid. Add 30 ppm SO2. Keep the container of crushed fruit in the vineyard to limit its exposure to commercial yeast in the winery and allow the native yeast fermentation to begin. Temperature of the starter should be kept around 26°C (by shading or sun exposure). Monitor the starter for Brix depletion and temperature daily (or twice per day if it is moving briskly). Oxygenate the starter after 2 or 3 days. When the starter is around 1.045 - 1.030 (between 8 and 12° Brix), strain the pomace in the bucket and keep the fermenting juice for inoculation. Make sure to smell and taste the starter before inoculation to be sure that VA and ethyl acetate are not too high.
References
(1) Green, J. A.; Parr, W. V.; Breitmeyer, J.; Valentin, D.; Sherlock, R. Sensory and Chemical Characterisation of Sauvignon Blanc Wine: Influence of Source of Origin. Food Research International 2011, 44 (9), 2788–2797.
(2) Benkwitz, F.; Tominaga, T.; Kilmartin, P. A.; Lund, C.; Wohlers, M.; Nicolau, L. Identifying the Chemical Composition Related to the Distinct Aroma Characteristics of New Zealand Sauvignon Blanc Wines. American Journal of Enology and Viticulture 2012, 63 (1), 62–72.
(3) des Gachons, C. P.; Leeuwen, C. V.; Tominaga, T.; Soyer, J.-P.; Gaudillère, J.-P.; Dubourdieu, D. Influence of Water and Nitrogen Deficit on Fruit Ripening and Aroma Potential of Vitis Vinifera L Cv Sauvignon Blanc in Field Conditions: Influence of Water and Nitrogen Deficit on Grape Aroma Potential. J. Sci. Food Agric. 2005, 85 (1), 73–85.
(4) Dubourdieu, D.; Tominaga, T.; Masneuf, I.; Gachons, C. P. des; Murat, M. L. The Role of Yeasts in Grape Flavor Development during Fermentation: The Example of Sauvignon Blanc. Am J Enol Vitic. 2006, 57 (1), 81–88.
(5) Larcher, R.; Tonidandel, L.; Román Villegas, T.; Nardin, T.; Fedrizzi, B.; Nicolini, G. Pre-Fermentation Addition of Grape Tannin Increases the Varietal Thiols Content in Wine. Food Chemistry 2015, 166, 56–61.
(6) Pinu, F. R.; Jouanneau, S.; Nicolau, L.; Gardner, R. C.; Villas-Boas, S. G. Concentrations of the Volatile Thiol 3-Mercaptohexanol in Sauvignon Blanc Wines: No Correlation with Juice Precursors. American Journal of Enology and Viticulture 2012, 63 (3), 407–412.
(7) Report | ARC by Laffort.
(8) Masneuf-Pomarède, I.; Mansour, C.; Murat, M.-L.; Tominaga, T.; Dubourdieu, D. Influence of Fermentation Temperature on Volatile Thiols Concentrations in Sauvignon Blanc Wines. Int. J. Food Microbiol. 2006, 108 (3), 385–390.
(9) Herbst-Johnstone, M.; Nicolau, L.; Kilmartin, P. A. Stability of Varietal Thiols in Commercial Sauvignon Blanc Wines. American Journal of Enology and Viticulture 2011, 62 (4), 495–502.
(10) Howell, K. S.; Klein, M.; Swiegers, J. H.; Hayasaka, Y.; Elsey, G. M.; Fleet, G. H.; Høj, P. B.; Pretorius, I. S.; de Barros Lopes, M. A. Genetic Determinants of Volatile-Thiol Release by Saccharomyces Cerevisiae during Wine Fermentation. AEM 2005, 71 (9), 5420–5426.
(11) Thibon, C.; Marullo, P.; Claisse, O.; Cullin, C.; Dubourdieu, D.; Tominaga, T. Nitrogen Catabolic Repression Controls the Release of Volatile Thiols by Saccharomyces Cerevisiae during Wine Fermentation. FEMS Yeast Research 2008, 8 (7), 1076–1086.
(12) O’Kennedy, K. Chitin, Chitinase, Chitosan ... Wineland Magazine, 2019.
(13) Malerba, M.; Cerana, R. Recent Advances of Chitosan Applications in Plants. Polymers 2018, 10 (2), 118.
(14) Barrett, L. Microbial Stability and Control: EnartisStab Micro (Chitosan) Application during Wine Maturation, 2019.
(15) Taillandier, P.; Joannis-Cassan, C.; Jentzer, J.-B.; Gautier, S.; Sieczkowski, N.; Granes, D.; Brandam, C. Effect of a Fungal Chitosan Preparation on Brettanomyces Bruxellensis, a Wine Contaminant. Journal of Applied Microbiology 2015, 118 (1), 123–131.
(16) Chung, Y.; Su, Y.; Chen, C.; Jia, G.; Wang, H.; Wu, J. C. G.; Lin, J. Relationship between Antibacterial Activity of Chitosan and Surface Characteristics of Cell Wall. Acta Pharmacol Sin 2004, 5.
(17) Chagas, R.; Monteiro, S.; Boavida Ferreira, R. Assessment of Potential Effects of Common Fining Agents Used for White Wine Protein Stabilization. American Journal of Enology and Viticulture 2012, 63 (4), 574–578.
