Controlling Microbes at Crush with Microcontrol and Egide in Chambourcin
Doug Fabbioli
Fabbioli Cellars
Summary
Grapes enter the winery covered in a host of microbes, some of whom contribute to complexity of flavor and aroma, while others may lead to spoilage. Fabbioli cold soaks red fermentations prior to inoculation under warmer than usual conditions (in the cellar rather than the cold room). The purpose of this experiment was to explore the use of chitosan and non-Saccharomyces yeast to use in addition to SO2 to keep the cold soaking TBins free of spoilage. Chambourcin grapes were destemmed and treated with either 25 mg/L SO2 alone, 25 mg/L SO2 + 10 g/hL Oenobrett, or 25 mg/L SO2 + 5 g/hL Egide. After completion of fermentation wine from each treatment had volatile acidity levels above 0.7 g/L, a generally accepted level of sensory perception. These values continued to increase during aging. Levels of acetic acid bacteria and other spoilage organisms were not notably different between treatments.
Introduction
At Fabbioli Cellars, Doug Fabbioli prefers to do a cold soak of red grapes prior to fermentation to extract color and complexity of flavor. However, he does not have adequate space in the refrigerated truck to store TBins undergoing cold soak. Instead, TBins are placed in the coolest portion of the cellar. Though cold soak can lead to positive effects, it is also a time when spoilage organsims such as Hanseniaspora uvarum can proliferate, leading to accumulation of ethyl acetate and acetic acid. The purpose of this experiment was to explore antimicrobial treatments to use in addition to SO2 to keep the cold soaking TBins free of spoilage.
Grapes enter the winery covered in microbes from the vineyard. To date, 52 different species of yeast from 22 different genera have been identified on grapes including Hanseniospora (AKA Klockera), Candida, Pichia, Hansenula, Metschnikowia, Sporoblomyses, Cryptococcus, Thodotorula, and Aurobasidium1. The cast of characters changes as grapes ripen, with the greatest abundance of microbes present in the last few weeks1. Healthy grapes are generally inhospitable environments for any microbe because they are covered in plates of wax that form a cuticle to hold in nutrition and repel water. Microbes cluster around the stomata or next to cracks in the cuticle where seepage from the openings provides both water and nutrients. The overall microbial load on grapes depends on environmental factors such as climate, altitude, variety, age of grapes, disease pressure and vineyard practices. Fog, rain, and fruit damage (like that seen in wet vintages like 2018) quickly transform the microbial desert of grape skins into an oasis. Cells that are present in small numbers quickly multiply when given the chance1. For example, Botrytis infection can increase the overall abundance of microbes by 1000x. Grapes with sour rot have significantly higher microbial diversity and abundance2. Insect pressure will also increase abundance due to increased vectoring from diverse environments3. The overall inoculant of non-Saccharomyces yeast and bacteria coming into the winery from the vineyard on the grapes is often larger than the inoculant of selected Saccharomyces yeast added at the beginning of fermentation1.
Non-Saccharomyces yeast have several impacts on the wine, both positive and negative. Klockera apiculata (aka Hanseniaspora uvarum) is a common member of the non-Saccharomyces yeast community found on grapes1,4. This yeast strain is easily identified under a microscope by its lemon shaped cells. It is tolerant to up to 100 mg/L SO2, can grow at low temperature (such as that found during cold soak), and can produce both acetic acid and ethyl acetate (which smells like nail polish remover) under aerobic conditions3. Other offenders in the non-Saccharomyces yeast community include Pichia guilliemondii, a film forming yeast prevalent in warm conditions when fermentation is delayed. This yeast can form spores that become resident in barrels and produce ethyl acetate and 4 ethyl phenol (which can smell like band-aid, wet dog, horse sweat)1–3.
Many spoilage bacteria also come into the winery on grapes. Sour rot and Botrytis increase the prevalence of Acetobacter, Gluconobacter and Gluconacetobacter, all of which produce acetic acid. Several Lactobacillus species (hilgardii, plantarum, casei) and Pediococcus (damnosus) are also residents of mature grapes. These can produce acetic acid, mousy flavor and biogenic amines (which have names like putrescine and cadaverine…). They may also produce polysaccharides that lead to ropy texture1,3,5.
In addition to outright spoilage, high levels of native flora may also cause nutrient depletion early in fermentations that limit nutrients available to Saccharomyces, potentially leading to stuck fermentations6,7. In a study of nutrient depletion by non-Saccharomyces yeast species, Medina et al (2012)6 found that Metchnikowia, a non-Saccharomyces yeast strain present in potentially high numbers on grapes8, consumed YAN quickly within the first few days of fermentation. Mimicking what may be occurring in fermentations with cold soaking or delayed inoculation, sequential inoculation with Metchnikowia followed by Saccharomyces led to stuck fermentations that could be resolved with nutrient addition. In the same study, Hanseniaspora, another prevalent member of the grape microbiota, did not show large YAN depletion (90% of the YAN remained 3 days after inoculation with this species), however it did deplete thiamine, an essential vitamin for Saccharomyces. Excessive use of SO2, as would occur in vintages with high microbial load, also leads to reduction in thiamine, further increasing the potential for stuck fermentations.
Despite the risks, there are also some benefits to having a rich microbial community early in fermentation. Several non-Saccharomyces yeast species have been shown to produce positive compounds that add complexity to wine aroma such as esters, higher alcohols, glycerol, succinic acid and thiols. Proteases produced by non-Saccharomyces yeast have been shown to break down cells and add nutrients, ultimately making a more protein stable wine. Some produce glycosidases that help unmask aromas compounds that are bound to sugar molecules. Others produce enzymes to break down polysaccharides that would otherwise inhibit clarification and filtration. Lachanacea thermotolerans has been shown to consume acetic acid, reducing volatile acidity1,8,9. It is likely these are some of the mechanisms that occasionally lead winemakers to employ ambient fermentations.
Many winemaking decisions affect the abundance and diversity of the microbial community present at the beginning of fermentation. Mechanical harvesting and long transport times, especially at warm temperatures, can lead to a high microbial load8,10. As soon as the grapes are crushed, nutrients are released to feed the organisms that are present. Klockera (Hanseniaspora) is often the most abundant species on the grapes , and remains prevalent until alcohol levels rise above 4-7% and oxygen is used up3,10. The low pH environment of the juice, rising alcohol, rising temperatures, and presence of phenolics tend to inhibit spoilage organisms in early fermentation. Harvesting wet grapes, prolonged cold soak, cool fermentation conditions, low inoculant of yeast, and lack of clarification (for white wines) can all lead to higher counts of yeast and bacteria in the fermentation3,11
In wet vintages such as 2018, the prevalence of damaged berries and wet grapes likely increased the inoculant of non-Saccharomyces microbes in fermentation and may have contributed to overall higher volatile acidity in wines that year. One approach to microbial management is to use higher than normal levels of SO2. Though SO2 has efficacy against some microbial spoilage, many of these microbes (such as Hanseniaspora) have high tolerance to it. Much of the SO2 added at crush is lost as it binds to grape solids that are prevalent in red wine fermentations, making it less effective. High SO2 additions can also bind thiamine and slow down or halt fermentation, and may even select for SO2 tolerant microbes that will cause spoilage during aging12,13. Still, fermentations that have some SO2 added at crush do tend to have faster onset of fermentation (leading to lower potential for spoilage) and steadier kinetics (Egli et al 1998).
When SO2 isn’t enough, or when you want to limit SO2 for other reasons, another option for combatting microbial spoilage during fermentation is chitosan. Chitosan is a naturally occurring molecule that can also be produced by the de-acetylation of chitin using NaOH or chitinase enzymes14. Chitin is the second-most common polymer found in nature (after cellulose), making up the cell walls of fungi and shells of crustaceans and thus readily available as a renewable resource5. In winemaking applications, chitin from Aspergillus nigeris used as the source for chitosan. The effectiveness of any given formulation of chitosan depends on its molecular weight, deacetylation degree and the pH of the medium15. Lower molecular weight, higher degree of deacetylation is the favored formulation for antimicrobial purposes16. At juice and wine pH, chitosan is very positively charged, which increases efficacy16.
Different microbes bind chitosan to different degrees. Chitosan binding to cell walls is driven by chemical properties of the cell wall itself, with high degree of correlation to the hydrophilicity of the wall17. Gram negative bacteria are more susceptible to binding than gram positive17. Chitosan has been shown to have some efficacy against a wide range of grapevine and wine microbes including downy mildew18, powdery mildew19, Phomopsis20, Lactobacillus, Oenococcus, and Brettanomyces17. Due to its versatility as an antimicrobial agent, chitosan in various forms has been used worldwide at nearly every stage of wine production including vineyard applications, on grapes during transport and storage, at crush, after fermentation and during the aging of wine16,21,22.
Many different mechanisms of antimicrobial action have been proposed for chitosan in wine. Chitosan has been shown to physically bind to the cell walls of microbes15,17. Binding may aid in sedimentation, leading to reduction in overall cell number with racking. Binding of the positively charged chitosan may also disrupt cell membranes, leading to leakage of ATP, potassium, and proteins, all essential components of cell function15,17. Leakage may therefore result in semi-viable cells or cell death. Other proposed mechanisms include the physical blocking of cell permeability by chitosan binding, chelation of survival factors such as copper, penetration of the cell membrane and binding to DNA15.
Regardless of the mechanism, chitosan has been shown to be quite effective in treating existing microbial infection of wine. When treating wine already inoculated with Brettanomyces bruxellensis, Taillander et al (2014) found that 85% of the population of Brettanomyces cells were dead within 20 hours of treatment. There was a dose effect in the rate of cell death, with 0.4 g/L treatment killing cells faster than 0.04 g/L. They also found differences in susceptibility based on the strain of Brettanomyces used, presumably due to genetic differences in cell wall components. Many cells initially compromised by chitosan treatment recovered, as evidenced by growth in the Brettanomyces population after 7 days. This rebound effect is a good argument for racking after treating an infection with chitosan. Other researchers tested the effect of 0.04 g/L chitosan on aging wine and found that, even at low dose, aging of wine on chitosan helped prevent infection by Brettanomyces23. Here, wine was not racked.
Most chitosan products are recommended for use in finished wine. OenoBrett is a chitosan and enzyme based preparation formulated for use in wine to combat Brettanomyces infection. In this experiment, 10 g/hL of OenoBrett was added to Chambourcin grapes at processing along with SO2.
Another addition to the toolkit for the prevention of microbial spoilage is to use a selected strain of bioprotective non-Saccharomyces yeast. Several strains of bioprotective yeast have been developed as commercially available products that inhibit the activity of spoilage organisms without depleting nutrients, undergoing fermentation, or inhibiting Saccharomyces. These strains are generally tolerant to SO2, so they can be used as an additional antimicrobial measure. Different strains use different mechanisms for bioprotection. One strain of Torulaspora delbrueckii has been shown to produce a killer toxin, TdKT, that uses ß-glycanase and chitinase activity to attack the cell walls of spoilage yeast24. Not all strains of T. delbrueckii tested had this activity, and if they did, it was not active against all of the soilage yeasts tested. Several strains of Metschnikowia pulcherrima have been shown to have broad and effective antimicrobial action against spoilage microbes. These employ pulcherriminic acid, which depletes iron from the surrounding medium to cause nutrient limitation. In addition, the presence of M. pulcherrima changed the gene expression of S. cerevisciae such that the fermentation yeast produced 40% less acetic acid and 12% more glycerol25.
Despite these promising results, it is important to keep in mind these bioprotectors cannot make up for poor cellar practices. James Osborne’s lab at Oregon State University conducted a series of experiments testing the ability of commercially available non-Saccharomyces yeast to reduce growth of Hanseniaspora uvarum and reduce the spoilage compounds made by this microbe (acetic acid and ethyl acetate). They used a model grape juice to grow mono-cultures and co-cultures of H. uvarum with several commercially available non-Saccharomyces yeast marketed as bioprotective (three Methchnikowia, three Lachanacea, and four Torulaspora). Each strain of bioprotective yeast decreased the level of acetic acid, and the number of H. uvarum cells present relative to mono-cultures of just H. uvarum. A single strain (M. fruticola) was further tested in Pinot noir must with and without cold soaking, at three levels of inoculation with H. uvarum. The number of H. uvarum cells decreased in cultures when H. uvarum was inoculated at low levels (1x103cells/mL) but not high levels (1x106 cells/mL). At all levels, inoculation with M. fructicola decreased ethyl acetate and acetic acid levels relative to control, but high levels of H uvarum still led to high levels of ethyl acetate (20-30 mg/L) and acetic acid (175-200 mg/L) produced during cold soak. After fermentation was complete, there were no significant differences in ethyl acetate or acetic acid between “no cold soak” control and cold soaked wines treated with M. fructicola. The take-home message here seems to be that though non-Saccharomyces yeast can help, they cannot overcome high levels of H. uvarum inoculant caused by damaged grapes or long cold soaking26.
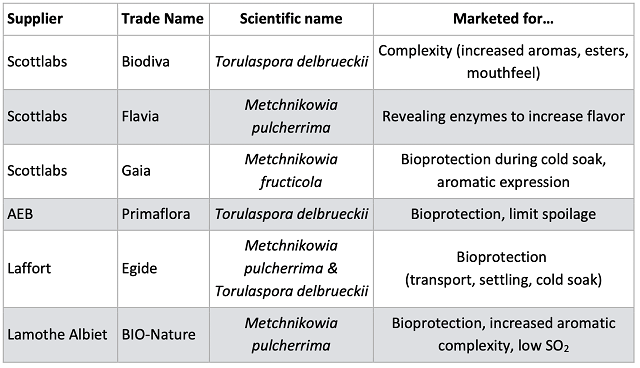
Several strains of non-Saccharomyces yeast are now commercially available for bioprotection as well as aroma/flavor enhancement (Table 1). In this experiment, Laffort Egide, a blend of Torulaspora delbrueckiiand Metschnikowia pulcherrima was added to Chambourcin grapes at processing. Both strains have been selected for organoleptic neutrality and good implantation. Chemical and microbiological measurements were taken for Chambourcin treated with SO2 alone, SO2 + 10 g/hL Oenobrett and SO2 + 5 g/hL Egide.
Table 1: Partial list of commercially available non-Saccharomyces yeast
Methods
At the time of harvest, fruit was divided evenly and randomly between three TBins (Control, Oenobrett Org, Egide). This fruit was intended for a three-day cold soak. However, Chambourcin fruit was picked in the morning of a 90°F day, then processed immediately without refrigeration. Fruit was destemmed and crushed into T-bins. Each bin received 25ppm SO2, 60 mL/ton Color Pro, and 1 lb/ton oak chips, mixed with a punchdown. There was no saignée for this experiment. Oenobrett (10 g/hL) and Egide (5 g/hL) were added by sprinkling directly onto the grapes during destemming. The Egide addition avoided SO2 addition by layering must in between. Tbins remained in ambient air until late afternoon, when bins were covered, gassed, and put in the cellar to cold soak for 3-5 days. The winemaker observed that the fruit had some volatile acidity at the time of processing. By 8AM the following morning, a cap had formed and Brix measurement indicated fermentation had begun. The bins were punched down and inoculated in the afternoon with 166g/ton D254 rehydrated in 208g/ton Go Ferm. Each T bin received 2 punchdowns per day during active fermentation. Each bin was pressed separately on the same day at the end of fermentation with no extended maceration, then inoculated with VP41 malolactic bacteria. At the completion of malolactic fermentation, 55ppm SO2 was added without racking.
Results
Fruit was harvested with 22.2°Brix of sugar at a pH of 3.38. By 3pm the following day, Brix was measured at 21.6° in the control, 22.0° in the Oenobrett treatment and 21.8° in the Egide treatment, with a temperature of 64°F. Neither OenoBrett nor Egide are intended to prevent the onset of fermentation. Fermentations progressed steadily and were pressed at the completion of alcoholic fermentation on Oct 10, for a total of 8 days after inoculation.
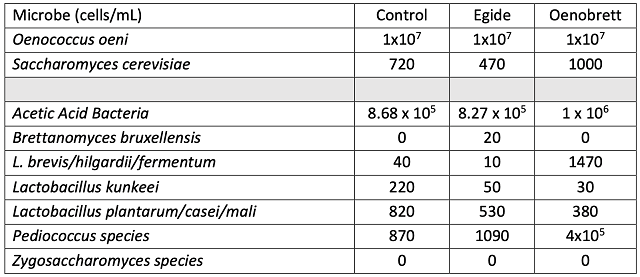
The control wine had slightly higher accumulation of volatile acidity after aging than either of the treatments, despite beginning with similar levels of VA (Table 2). All three of the treatments show residual sugar, indicating future danger for spoilage organisms. When evaluating differences in microbiological load, a difference of 10x is generally considered notable. Levels of spoilage organisms differ among treatments, but without clear patterns (Table 4). Levels of potential spoilage organisms in the Oenobrett barrel may be due to the effects of the barrel rather than those of the treatment. As demonstrated by Osborne26, no single antimicrobial intervention seems to be enough to prevent spoilage. Rather, several practices should be stacked together to present enough hurdles that microbes cannot overcome them.
Table 2: General chemistry for three treatments of Chambourcin (ICV labs)
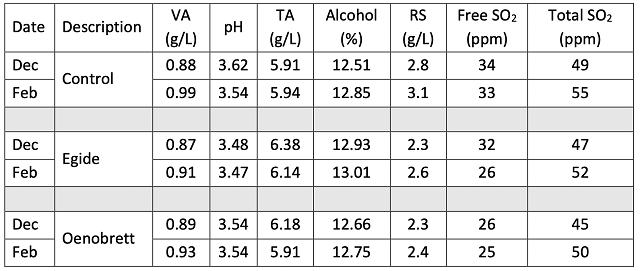
Table 3: Color metrics for three treatments of Chambourcin (ICV labs)
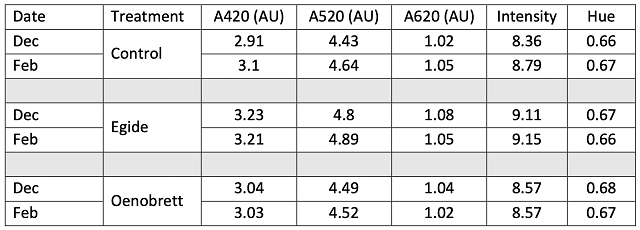
Table 4: Microbiology of three treatments of Chambourcin (ETS labs)
References
(1) Jackson, R. S. Wine Science: Principles and Applications, 4 edition.; Academic Press: Amsterdam, 2014.
(2) Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The Microbial Ecology of Wine Grape Berries. International Journal of Food Microbiology 2012, 153 (3), 243–259.
(3) Wine Microbiology. UC Davis Viticulture and Enology.
(4) Diversity of Wine Yeasts | Viticulture and Enology https://wineserver.ucdavis.edu/industry-info/enology/wine-microbiology/yeast-mold/diversity-wine-yeasts (accessed 2018 -11 -13).
(5) García, M. E. R.; Esteve-Zarzoso, B.; Arroyo, T. Non-Saccharomyces Yeasts: Biotechnological Role for Wine Production; 2016.
(6) Medina, K.; Boido, E.; Dellacassa, E.; Carrau, F. Growth of Non-Saccharomyces Yeasts Affects Nutrient Availability for Saccharomyces Cerevisiae during Wine Fermentation.
(7) Bisson, L. F. Stuck and Sluggish Fermentations. American Journal of Enology and Viticulture 1999, 50(1), 107–119.
(8) Jolly, N. P.; Varela, C.; Pretorius, I. S. Not Your Ordinary Yeast: Non- Saccharomyces Yeasts in Wine Production Uncovered. FEMS Yeast Res 2014, 14 (2), 215–237.
(9) Vilela, A. Lachancea Thermotolerans, the Non-Saccharomyces Yeast That Reduces the Volatile Acidity of Wines. Fermentation 2018, 4 (3), 56.
(10) Ciani, M.; Domizio, P. Controlled Mixed Culture Fermentation: A New Perspective on the Use of Non- Saccharomyces Yeasts in Winemaking. FEMS Yeast Research.
(11) Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled Mixed Culture Fermentation: A New Perspective on the Use of Non-Saccharomyces Yeasts in Winemaking. FEMS Yeast Res. 2010, 10 (2), 123–133.
(12) Ndlovu, T.; Divol, B.; Bauer, F. F. Yeast Cell Wall Chitin Reduces Wine Haze Formation. Appl. Environ. Microbiol. 2018, 84 (13), e00668-18.
(13) Ciani, M.; Comitini, F. Yeast Interactions in Multi-Starter Wine Fermentation. Current Opinion in Food Science 1, 1–6.
(14) O’Kennedy, K. Chitin, Chitinase, Chitosan ... Wineland Magazine, 2019.
(15) Taillandier, P.; Joannis‐Cassan, C.; Jentzer, J.-B.; Gautier, S.; Sieczkowski, N.; Granes, D.; Brandam, C. Effect of a Fungal Chitosan Preparation on Brettanomyces Bruxellensis, a Wine Contaminant. Journal of Applied Microbiology 2015, 118 (1), 123–131.
(16) Barrett, L. Microbial Stability and Control: EnartisStab Micro (Chitosan) Application during Wine Maturation, 2019.
(17) Chung, Y.; Su, Y.; Chen, C.; Jia, G.; Wang, H.; Wu, J. C. G.; Lin, J. Relationship between Antibacterial Activity of Chitosan and Surface Characteristics of Cell Wall. Acta Pharmacol Sin 2004, 5.
(18) Romanazzi, G.; Mancini, V.; Feliziani, E.; Servili, A.; Endeshaw, S.; Neri, D. Impact of Alternative Fungicides on Grape Downy Mildew Control and Vine Growth and Development. Plant Disease 2016, 100 (4), 739–748.
(19) Iriti, M.; Vitalini, S.; Di Tommaso, G.; D’Amico, S.; Borgo, M.; Faoro, F. New Chitosan Formulation Prevents Grapevine Powdery Mildew Infection and Improves Polyphenol Content and Free Radical Scavenging Activity of Grape and Wine: Grape Powdery Mildew Control by Chitosan. Australian Journal of Grape and Wine Research 2011, 17 (2), 263–269.
(20) Nascimento, T.; Rego, C.; Oliveira, H. Potential Use of Chitosan in the Control of Grapevine Trunk Diseases. Phytopathologia Mediterranea 2007, 46 (2), 8.
(21) Singh, R. K.; Martins, V.; Soares, B.; Castro, I.; Falco, V. Chitosan Application in Vineyards (Vitis Vinifera L. Cv. Tinto Cão) Induces Accumulation of Anthocyanins and Other Phenolics in Berries, Mediated by Modifications in the Transcription of Secondary Metabolism Genes. IJMS 2020, 21 (1), 306.
(22) Singh; Soares; Goufo; Castro; Cosme; Pinto-Sintra; Inês; Oliveira; Falco. Chitosan Upregulates the Genes of the ROS Pathway and Enhances the Antioxidant Potential of Grape (Vitis Vinifera L. ‘Touriga Franca’ and ’Tinto Cão’) Tissues. Antioxidants 2019, 8 (11), 525.
(23) Bağder Elmaci, S.; Gülgör, G.; Tokatli, M.; Erten, H.; İşci, A.; Özçelik, F. Effectiveness of Chitosan against Wine-Related Microorganisms. Antonie Van Leeuwenhoek 2015, 107 (3), 675–686.
(24) Villalba, M. L.; Susana Sáez, J.; del Monaco, S.; Lopes, C. A.; Sangorrín, M. P. TdKT, a New Killer Toxin Produced by Torulaspora Delbrueckii Effective against Wine Spoilage Yeasts. International Journal of Food Microbiology 2016, 217, 94–100.
(25) Sadoudi, M.; Rousseaux, S.; David, V.; Alexandre, H.; Tourdot-Maréchal, R. Metschnikowia Pulcherrima Influences the Expression of Genes Involved in PDH Bypass and Glyceropyruvic Fermentation in Saccharomyces Cerevisiae. Front. Microbiol. 2017, 8.
(26) Osborne, J. Yeast Management for Stressful Alcoholic Fermentation, 2019.
