Considerations for Co-Inoculation
Joy Ting
August 2024
Do I need to inoculate for malolactic fermentation?
After the completion of alcoholic fermentation, most red wines undergo malolactic fermentation, a process that stabilizes the wine against microbial attack during aging. This process also shifts the perception of acidity by lowering the TA (and increasing pH) of the wine1. Malolactic fermentation itself includes the removal of carbon dioxide from malic acid, leaving lactic acid behind. Lactic acid bacteria (LAB) harness the energy released from breaking this bond and apply it to energy consuming processes in the cell. Many different genera of bacteria contain the enzymes needed to decarboxylate malic acid2. In addition to malolactic fermentation, these microbes usually have many other impacts on the sensory properties of the wine, both positive and negative3.
Oenococcus oeni is the main malic acid consuming bacteria in winery settings. This bacteria is able to survive in the high alcohol, low pH, low nutrient conditions of wine, and still complete malolactic fermentation with a minimal level of spoilage characteristics1. Though O. oeni is very prevalent in wineries, it is not the most common LAB found on grapes. Many different species of LAB, including Lactobacillus and Pediococcus, are found on grapes in higher population density than Oenococcus oeni. Oenococcus oeni is often so scarce in grape samples that enrichment techniques are needed to detect them4. As fermentation proceeds, alcohol accumulates and oxygen becomes scarce, leading to a decline in population level of other LAB whie O. oeni begins to dominate. By the end of alcoholic fermentation, the population level of Lactobacillus and Pediococcus is low while O. oeni is poised to drive malolactic fermentation3.
If grapes are healthy (with limited LAB) and the winery has been through several vintages, there may be enough O. oeni resident to complete malolactic fermentation without inoculation. However, if grapes are compromised when they enter the winery, spoilage LAB will be more concentrated. Wine pH higher than 3.5 is more permissive/less inhibitory to LAB3,4. If LAB other than O. oeni predominate, the wine is vulnerable to spoilage characteristics such as volatile acidity (acetic acid and ethyl acetate), mouse taint, biogenic amines, and ropy texture5. Inoculation help O. oeni to predominate and limits the impact of other LAB.
Does it matter which ML bacteria I use?
The genome of Oenococcus oeni contains around 1700 genes, roughly half of which are known to be variable within the species. This means that different strains may have different levels of tolerance, metabolic rate, and production levels of secondary metabolites3. One of the most familiar sensory outcomes of malolactic fermentation is the production of diacetyl, the compound that smells like movie theater popcorn (“butter”). Diacetyl production differs by strain (as well as environmental factors), as does production of fruity esters (red berry character), liberation of oak lactones (vanilla) from barrels, and degradation of vegetal aromas3. One only needs to look at recent versions of product catalogues to see full aroma profiles from different strains of bacteria.
The strain of LAB that completes malolactic fermentation may also alter the impact of future Brettanomyces infections. Some strains of Oenococcus oeni (and other LAB) have enzymes that release tartaric acid bound to hydroxycinnamic acids (TAE-HCA’s), releasing p-coumaric and ferulic acids. Brettanomyces bruxellensis use p-coumaric and ferulic acids as substrate to produce 4EP and 4EG, the compounds responsible for barnyard, spice and clove impacts in wine. Brettanomyces bruxellensis is unable to degrade TAE-HCAs on its own, so the production of this substrate by malic acid bacteria can greatly increase the concentration of 4EP and 3EG in Brett infected wines. Not all strains of Oenococcus oeni contain these enzymes6. Commercial strains have been tested and removed from the market if they have the enzymes6, but ambient strains do not have this guarantee.
Why is my malolactic fermentation so slow this year?
(...and how might co-inoculation help?)
Though Oenococcus oeni is (thankfully) better adapted to the harsh conditions of wine than other LAB, it is still more likely to be inhibited by environmental conditions than Saccharomyces cerevisiae. Stress is cumulative; though environmental conditions may be within the tolerance limits on their own, if two or three conditions are moderately stressful (close to the edge of the range), then bacteria may still be inhibited or even killed. Some manufacturers now offer a scorecard to add up potential stresses to determine if specialized bacteria may be needed.
Potential stressors include:
- Ethanol – Bacterial cell membranes are less complex than Saccharomyces membranes. As ethanol concentration rises, membranes become less fluid, limiting entry of nutrients into the cell. They also become more permeable, leading to destabilization1.
- Temperature - If the temperature is too low, metabolic processes occur very slowly. If temperature is too high, membranes become too permeable, adding to stress already exerted by high ethanol. Spoilage microbes are also more active at higher temperatures, leading to competitive inhibition for O. oeni1.
- pH – If pH is too high (>3.6), spoilage microbes are less inhibited, leading to competitive inhibition. If the pH is too low, even small amounts of free SO2 leads to inhibitory concentration of molecular SO2. Oenococcus can be inhibited by as little as 0.1 – 0.15 mg/L of molecular SO2, while levels from 0.3 – 0.5 mg/L can be toxic7. At a pH of 3.6, free SO2 of 6-9 mg/L of meets the inhibition threshold while free SO2 of 19-31 mg/L would be toxic.
- Total SO2 – When SO2 is added to grapes or juice during processing, most of it is quickly bound up by sugars. As fermentation proceeds, yeast produce acetaldehyde, which binds SO2 even more strongly. Between sugars and acetaldehyde binding, free SO2 is nearly zero. Oenococcus oeni metabolize acetaldehyde, releasing bound SO2 to the free fraction. If the pH is low enough, the molecular SO2 may each inhibitory concentration. In this way, all of the SO2 has the potential to form toxic products for malic acid bacteria7.
- Yeast strain – The total SO2 of a fermenting wine includes any SO2 added at crush plus SO2 produced by the yeast. Most Saccharomyces strains produce some SO2 during fermentation, with the amount dependent on conditions. One survey of SO2 production by four different strains of commercial Saccharomyces found a range of 13-42 ppm SO2 produced under the same conditions8. In the product catalogue, high SO2 producing yeast are often noted to be less permissive of malolactic fermentation.
- Nutrients – Like other microbes, Oenococcus oeni needs a source of nitrogen to build proteins. Unlike Saccharomyces, they do not generate amino acids on their own, so adding DAP does not help. They also cannot take up amino acids from the environment, but rather they take up small proteins to supply this need. For these reasons, YAN is not a good indicator if the juice/wine contains ample nutrients for malolactic bacteria to grow and metabolize. A good rule of thumb is that if the alcoholic fermentation was stressful for the yeast, then nutrients might be limiting. As yeast cells break down in the lees, they release needed peptides7. Options for supplementation specific to malolactic bacteria are also available from vendors.
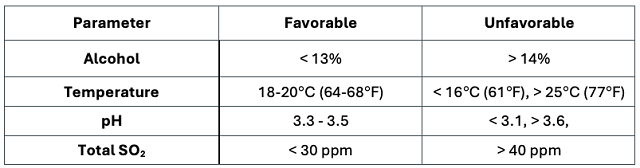
Table 1: Favorable and unfavorable conditions for malolactic fermentation (adapted from AWRI: Achieving Successful Malolactic Fermentation1)
What are the pros and cons of co-inoculation?
Co-inoculation allows malic acid bacteria to avoid many of the most inhibitory of the conditions listed above. Despite the name, co-inoculation of malic acid bacteria usually occurs 12-48 hours after yeast inoculation1,9. If added at the same time as yeast inoculation, malic bacteria loose 99% of their viable cells due to free sulfites8. As fermentation begins, yeast produce compounds to detoxify SO2, allowing a much more permissive environment for malic acid bacteria. When added early, bacteria experience a low ethanol, high nutrient environment with the lowest pH the wine will likely have. Bacterial action is usually suppressed during active fermentation, but cells still acclimatize their cell membranes to rising alcohol levels during this time. As the yeast start to die off at the end of fermentation, they release nutrients back into the wine just as malic bacteria transition from lag phase to log phase9. When malolactic fermentation occurs under the reductive conditions of fermentation, diacetyl production is low and lower pH leads to higher production of fruity esters.
Unfortunately, most malic acid bacteria, including Oenococcus oeni are heterofermentative, which means that they possess the metabolic machinery to utilize glucose and fructose to gain energy with acetic acid as a byproduct2. Malic acid and citric acid are consumed preferentially over sugar, however, if these substrates have been depleted, malic acid bacteria will consume any sugar that remains. In a normal fermentation, sugar is consumed before malic acid is fully depleted. However, in a stuck or sluggish fermentation, or in whole cluster fermentation that releases sugar at pressing, residual sugar will result in production of high amounts of acetic acid. Careful fermentation monitoring is key to success with coinoculation3. Malic acid bacteria are also more sensitive to high temperature, so in highly extractive, hot fermentations, sequential inoculation will be more successful.
References
(1) Achieving Successful Malolactic Fermentation, 2020. https://www.awri.com.au/wp-content/uploads/2011/06/Malolactic-fermentation.pdf.
(2) Liu, S.-Q. Malolactic Fermentation in Wine - beyond Deacidification. J Appl Microbiol 2002, 92 (4), 589–601.
(3) Krieger-Webe, S. Good Management of Malolactic Fermentation - Sensory Impacts, 2024.
(4) Liu, Y.; Rousseaux, S.; Tourdot-Maréchal, R.; Sadoudi, M.; Gougeon, R.; Schmitt-Kopplin, P.; Alexandre, H. Wine Microbiome: A Dynamic World of Microbial Interactions. Critical Reviews in Food Science and Nutrition 2017, 57 (4), 856–873.
(5) ETS Labs. Scorpions Bacteria Panel. https://www.etslabs.com/analyses/analysis/161987.
(6) Chescheir, S.; Philbin, D.; Osborne, J. P. Impact of Oenococcus Oeni on Wine Hydroxycinnamic Acids and Volatile Phenol Production by Brettanomyces Bruxellensis. American Journal of Enology and Viticulture 2015, 66 (3), 357–362.
(7) Malolactic Fermentation: Importance of Wine Lactic Acid Bacteria in Winemaking; Morenzoni, R., Ed.; Lallemand Inc.: Montreal, 2015.
(8) Henick‐Kling, T.; Park, Y. H. Considerations for the Use of Yeast and Bacterial Starter Cultures: SO2 and Timing of Inoculation. American Journal of Enology and Viticulture 1994, 45 (3), 464–469.
(9) du Toit, M. The Wine Expert: Co-Inoculation of Selected Wine Bacteria, n.d.
