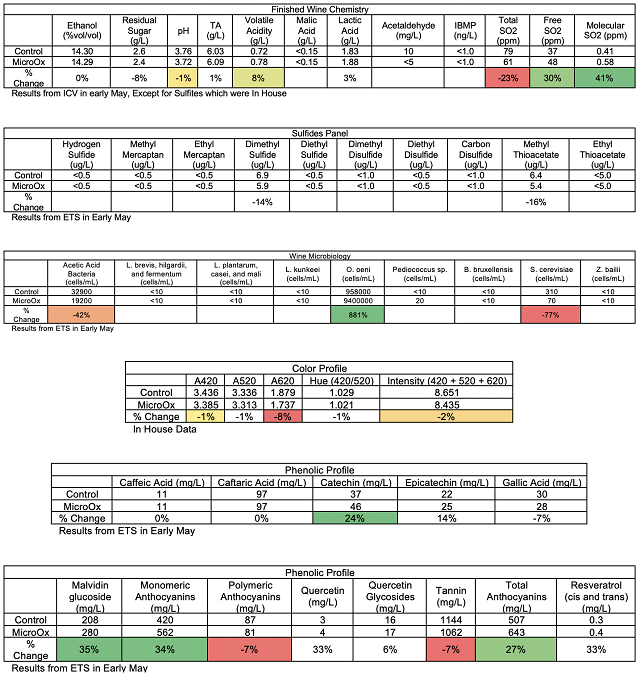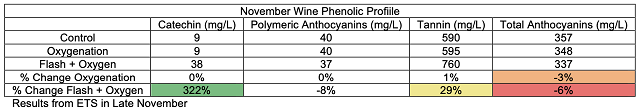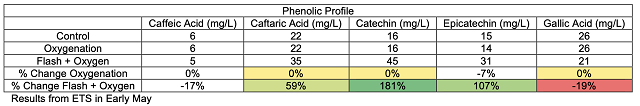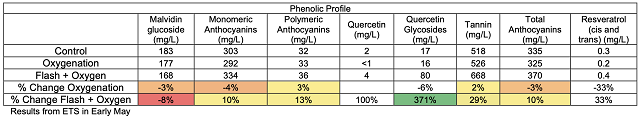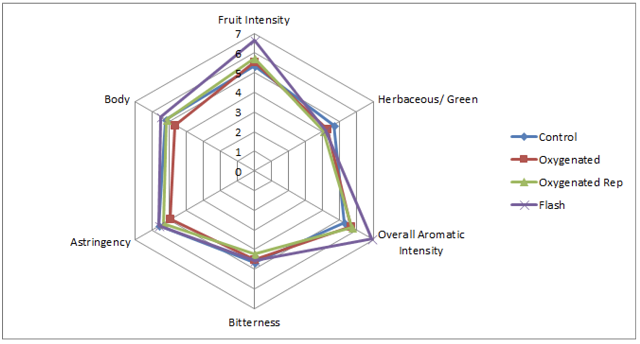
from La micro-ogxygenation ou micrbullage, wine-note.com”
Uptake of oxygen during normal winemaking operations
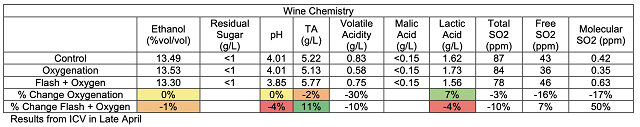
There are several steps during the winemaking process when oxygen is introduced into juice and wine. Oxygen is introduced almost every time the wine is moved and even during storage (Table 1). After introduction to the wine, oxygen is quickly bound by various components of the wine including ethanol (to form acetaldehyde), phenolics (tannins and anthocyanins), SO2, aroma and flavor compounds, and can have a profound effect on astringency and aroma. When a red wine (at 20°C) is saturated with oxygen, it contains an average of 6.3 -8.4 mg/L.1,2 Due to oxygen binding with other compounds in the wine , this level reduced to only 1 mg/L within 6 days3. During the course of red winemaking at least 10 saturations are beneficial for the evolution of the wine, while most red wines can tolerate at least 30 saturations, depending on their phenolic content2.
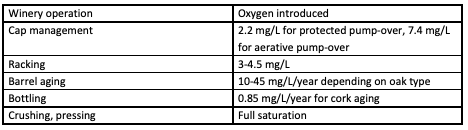
Table 1: Oxygen introduction during winemaking operations1,3
Unlike the uncontrolled addition of oxygen during cellar operations, micro-oxygenation (MOX) is the process of intentionally adding small amounts of oxygen in a measured way to induce favorable changes in color, aroma, and texture4. The technique was first developed in France in the 1990’s as a way of aging of tannic red wines in stainless steel tanks rather than oak barrels. It is now also used to accelerate aging, stabilize color, and treat reduction and herbaceous/green character. Rather than shortening the life of a wine, as oxidation normally does, MOX can increase the anti-oxidative power and potential aging of the wine by stimulating phenolic reactivity1.
Chemical and Sensory effects of micro-oxygenation
When oxygen is introduced to wine, it diffuses randomly and encounters many chemical compounds in the wine. Oxygen is generally highly reactive and will show the most effects in those chemicals that are found in the highest concentration in wine, such as ethanol, anthocyanins, tannin subunits, and tannins themselves. Oxygen will also interact with molecules that are responsible for aroma and flavor. Though a winemaker may be targeting one component, oxygen will interact with them all.
One of the most common reasons to micro-oxygenate wine is to help structure tannins. Phenolics such as anthocyanins and tannins are responsible for the color and mouthfeel (astringency) of red wine. The full chemistry of oxygen and phenolics in wine is complex and beyond the scope of this newsletter, however, a few details might aid in understanding the effect of MOX on astringency and color stability.
Tannins themselves are polymers, large molecules composed of smaller subunits that are bonded together in a specific way. After tannin polymers are extracted from the skins and seeds of grapes, acid hydrolysis in the grape juice and wine breaks these large polymers into smaller polymers and monomers. Phenolic monomers can exist in different oxidation states at wine pH, some of which are more reactive than others. The phenolic compounds extracted from grape skins and seeds are generally not very reactive in the wine matrix. However, when oxygen is introduced to the wine, it reacts with metals to start a chain reaction that leads to the activation of the monomers, making them more likely to react with other monomers to reassemble tannin monomers into polymers. Each time two monomers (or a monomer and growing chain) bond, they become even more likely to bond again, leading to elongation of the tannin polymer. Put simply, activation by oxygen can begin a cascade that results in faster formations of phenolic polymers. These chains continue to grow, adding more phenolic subunits, until they bond with an anthocyanin. When an anthocyanin is added to the chain, it is not likely to bind with anything else, effectively capping the chain2,3,5.
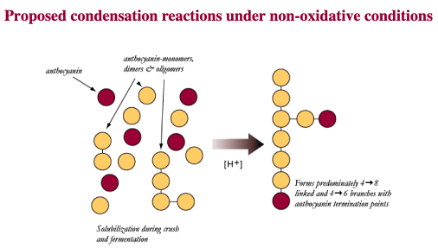
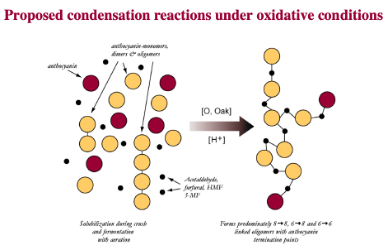
Figure 1: Products of condensation reactions among tannin subunits differ based on the presence of oxygen. From: Micro-oxygenation A Treatsise (McCord)6”
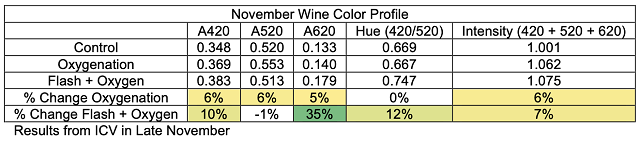
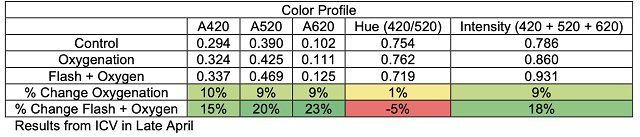
These chain reactions of tannin molecules have important effects for the color and astringency of the wine. The perceived mouthfeel of tannins is, in part, related to the length of the phenolic chains. Longer chains have more reactive sites for interaction with salivary proteins, thus the higher the degree of polymerization, the more astringent tannins seem. Micro-oxygenation soon after fermentation is thought to bind anthocyanins to growing chains before they are lost to precipitation, enzymatic attack or binding to lees, resulting in better color retention and shorter tannin polymers1,7. Smith (2014) also claims that smaller polymeric chains form a finer-grained colloidal structure, leading to better flavor integration7. Polymeric pigments (tannin polymers capped with anthocyanins) are less susceptible to oxidation and browning than monomeric anthocyanins, so MOX also aids in in color stability by preserving anthocyanins in solution in their colored form3.
Micro-oxygenation also affects tannin perception through a side reaction with acetaldehyde.
When oxygen reacts with metals in the wine, it produces hydrogen peroxide which interacts with ethanol to form acetaldehyde. Acetaldehyde binds with anthocyanins, potentially forming more stable color molecules. It also allows bridging between tannin molecules that may alter the sensory characteristics of wine3. Specifically, cross-linked tannins have a more globular structure, and therefore less astringency. Some cross-linked tannins are prone to precipitate, further decreasing astringency4, but this would also lead to loss of color.
In addition to color stability and tannin structure, micro-oxygenation also has an effect on the perception of reduced compounds such as H2S (sewer gas, rotten eggs) and methyl mercaptan (cabbage, skunk). Reduction is the chemical state of a compound whereby it has gained electrons in a reaction. Hydrogen atoms only hold their electrons weakly, so when they are bonded to sulfur atoms, the electrons spend more time near the sulfur, resulting in a reduced sulfur compound. Oxygen atoms on the other hand are strong electron attracters, so when oxygen is added to a solution with reduced sulfur compounds, some of the electrons will be attracted to the oxygen atoms and leave the sulfur atom, thereby oxidizing the compound. Oxidation of hydrogen sulfide results in two odorless compounds (water and elemental sulfur). Oxidation of methyl mercaptan results in dimethyl sulfide, which still has off odors of onion and garlic, but at a higher threshold of detection (12 ppb vs. 0.2-2 ppb)1,8.
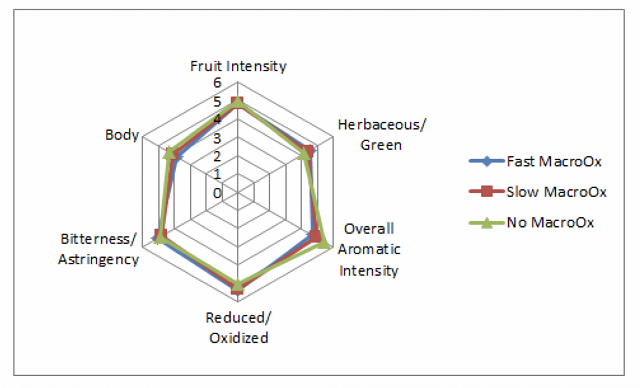
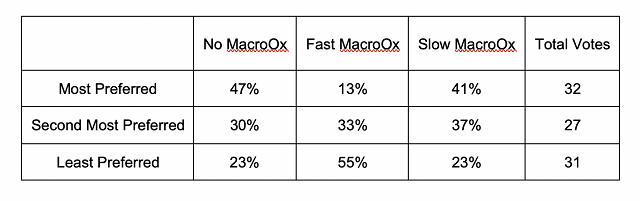
When it was first introduced, reports showed that micro-oxygenation also led to significant decreases in the sensory perception of herbal/vegetal character in wine. However, repeated measurement of the chemical methoxypyrazine, a principle chemical component of “veggie”, did not change. Further study revealed that the perception of “veggie” included a combination of pyrazine as well as some reduced sulfur compounds (AKA thiols) with vegetal aromas (such as canned green beans). The reduction of the perception of “veggie” after micro-oxygenation was due to oxidation of these reduced compounds8,9.
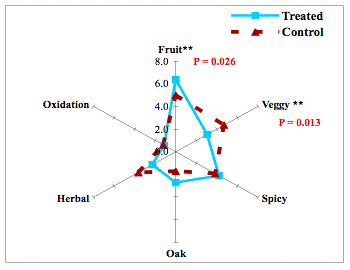
Figure 2: from Enology Notes #134, Zoecklein (2007)9
Risks of micro-oxygenation
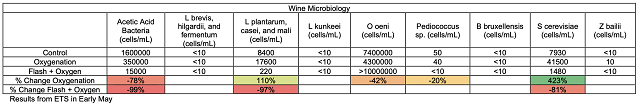
Despite its potential benefits, micro-oxygenation has some risks. Allowing micro-oxygenation to go on too long can lead to excessive polymerization, making tannins dry and harsh8. Oxygenation at too high of a rate can cut off the chain reaction of polymerization by starting too many chains at once. Ultimately this leaves excess oxygen in the wine that reacts with anthocyanins to cause browning as well as varietal aromas and flavors, causing oxidative off odors2. Oxygen availability in wine can also lead to microbial spoilage. Acetobacter will use available oxygen to produce acetic acid. Brettanomyces growth is facilitated by the presence of oxygen8(though it is thought that at low levels, micro-oxygenation poses less of a threat than standard racking4). Zoecklein 1 likens the management of micro-oxygenation to piloting an ocean liner. It must be done carefully and pro-actively with the understanding that one action begets a chain reaction that is not easy to control or fully predict. A firm understanding of the underlying mechanisms at play and careful monitoring of progress are key to success.
How and when to micro-oxygenate
To micro-oxygenate wine, an oxygen supply (purchased from the gas supplier) is connected to a dosing chamber (such as the Stavin Ox Box). The dosing chamber acts as a high precision dosing regulator that can be set for very small amounts of oxygen flow. Oxygen exits the box through small tubing into miniature sparging tips with very small (10 um) pore size to produce very fine bubbles6. Soluble solids and lees have been shown to interfere with MOX effects10, and can contain spoilage microbes, so the tubing is configured so that the sparging tips are suspected about an inch above the bottom of the tank to avoid disturbing any remaining lees.
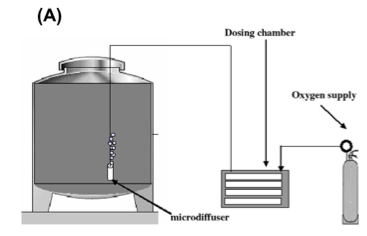
Figure 3: Schematic representation of a micro-oxygenation system. From Gomez-Plaza and Cano-Lopez (2010)4
MOX can be applied at any time during vinification and maturation, with total doses up to 600 mL/L for tannic red wines over the lifetime of the wine10. The effects of MOX will differ depending on the timing and dose rate of the addition.
Mox has been used during fermentation as a means to encourage yeast health and reduce off aromas during fermentation.
MOX prior to malolactic fermentation (MLF) has been shown to be effective in improving wine structure. Oxygen addition at this stage stimulates polymerization when tannins are most susceptible10. Shortly after the completion of alcoholic fermentation, anthocyanins and tannins are still in monomeric form, and thus in greatest need of oxygen for polymerization. Wine has greater reductive strength at this time since many potential binding sites for oxygen remain un-reacted. Dosage rates before malolactic fermentation can be 10 times higher than those after MLF, within a range of 20-60 mg/L/month for two to six weeks10,11. MLF can be delayed with the addition of lysozyme or chitosan to allow enough time for MOX to proceed.
After malolactic fermentation, it is thought that at least some polymerization has already occurred, so MOX has less of an effect on tannin structure. In addition, at this point, some anthocyanins have been lost to enzymatic processes and interactions with lees. Due to the lesser number of free anthocyanins and monomeric phenols, lower doses of oxygen (1-5 mg/L/month for up to 3 months) are used at this stage. In addition, the threat of acetaldehyde accumulation is greater. Malolactic bacteria consume acetaldehyde, so accumulation after alcoholic fermentation is somewhat moderated by consumption at the end of malolactic fermentation. After MLF is complete, this is no longer an option. However, due to the high number of wines needing attention during harvest, applying MOX after MLF may be more practical. It is not uncommon for MOX to be done at higher rates before malolactic fermentation, stopped while malic acid bacteria are active, then applied again at lower rates after malic acid has been depleted4,10.
In addition to timing and dosage of oxygen addition, several other considerations come into play when planning micro-oxygenation treatment:
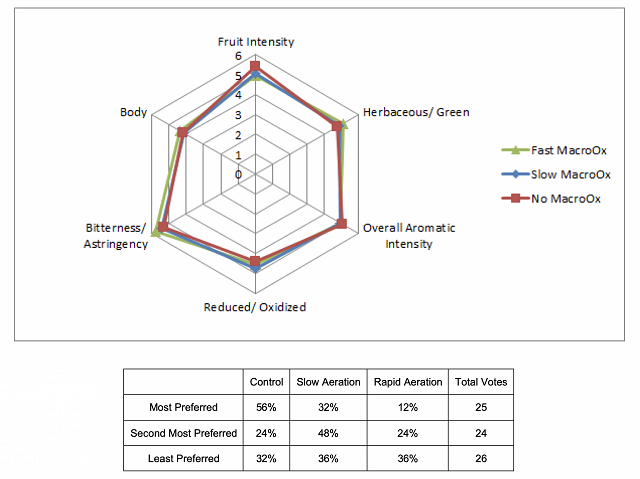
Continuous vs. discontinuous: Supplying a higher rate of oxygen all at once may not have the same effect as supplying it over the entire course of the month. Singleton (1987) found that “slow oxidation does not exhaust the original oxidizable substrates of wine as rapidly as does fast oxidation”2. Practically, this means that slow oxidation gives wine greater reductive strength, making it less susceptible to oxidation later while avoiding oxidation of flavor and aroma compounds as well as color1,2. Slow delivery also allows the utilization of one day’s oxygen prior to addition of the next day’s dose12. For these reasons, constant oxygenation is safer than discontinuous application since flow rates are slower and monitoring is easier to follow.
Based on the flow rates of the equipment, however, it may not be possible to dose the wine continuously, especially in the post-MLF treatment. If using the Stavin Ox Box (as in the experiments conducted at Michael Shaps Wineworks), the meter on the box cannot measure below 0.25 ml/L/minute. For a tank containing 2100 L (555 gallons) of wine, the box would need to be set at 0.15 mL/minute to achieve a target median post-ML flow rate of 3 mg O2/L/month6. It is possible to deliver this flow rate in an episodic manner instead, dosing at a higher dose for less time to hit the same overall target dose. Periodic oxygenation is closer to what wine would experience by standard aging in a barrel6.
Path height: A minimum path length of 2.2 – 4 m (depending on the dosage) is required in order to fully dissolve oxygen in the wine and not increase oxygen in the headspace using a traditional MOX unit13. Barrel adaptations have been made that allow MOX with a shorter path length.
Temperature: Temperature is an important variable for MOX. Warmer temperature leads to lower solubility of oxygen and more buildup in the headspace. Colder temperature slows down oxidation and polymerization reactions. A temperature near 15°C (within a range of 14-17°C ) is recommended as a good compromise10.
Turbidity: Soluble solids and lees can contain spoilage microbes such as Acetobacter and Brettanomyces that will consume oxygen and produce off flavors and odors. Wines should be racked carefully off lees with a target of <100 NTU prior to treatment10.
SO2 level: SO2 will somewhat slow the effects of MOX14, but it will also help protect the wine from severe oxidation10. If SO2 is present, it should not exceed 25 ppm free SO2.
Monitoring the progress of micro-oxygenation
Micro-oxygenation follows the goldilocks principle. If you do not add enough oxygen you will not see the benefits. If you push it too far, you can end up with dry tannins and oxidized wine. How can you tell when your oxygen dose and duration are “just right”? There are several measurements for monitoring the progress of MOX.
Dissolved oxygen (DO) is a useful monitoring tool, as long as the measurement is taken from the tank without introducing additional oxygen. If the dosage of oxygen is set correctly, most of the oxygen should be bound by phenolics rather than remaining dissolved or filling the headspace. High DO rates indicate MOX is set too high for the wine. Measuring DO before and after treatment can give an indication if the dose rate is too high10.
Volatile acidity (VA) is another endpoint used to monitor progress. Acetic acid bacteria utilize excess DO to produce acetic acid, causing a spike in VA. This indicates that oxygen dose rate is too high and is a warning sign of wine spoilage.
Acetaldehyde buildup will also indicate that oxygen rates may be too high. Enzymatic kits for testing acetaldehyde using a spectrophotometer can be purchased from enological companies (for example, Unitech sells 20 tests for $65.00). If a spectrophotometer is not available, McCord6 suggests a test comparing a fresh glass of wine with one that has been sitting out covered by a watchglass overnight. Excess acetaldehyde has sensory effects of rotten apples or a sherry-like quality15. If acetaldehyde is detected in both samples, then the rate of oxygenation is too high. If the overnight sample smells of pumpkin or chocolate, but the fresh sample does not, the rate is good. If there is no difference between the two glasses, then the rate should be increased.
In wines treated with micro-oxygenation after malolactic fermentation, free SO2 has been found to be an effective monitoring tool. SO2 rates should stay above 10 ppm to avoid oxidation4. If SO2 decreases too rapidly, then the O2 dose is too high.
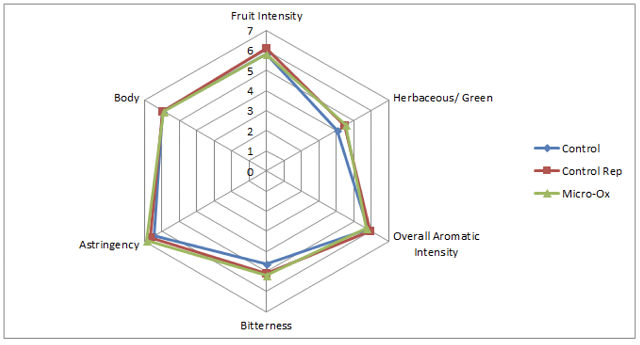
Careful sensory analysis throughout the experiment is a key tool to determining if acetaldehyde is building up, if Brettanomyces or Acetobacter have taken hold, if reduction is diminishing, and how the tannins are evolving.
References
(1) Zoecklein, B. W. Winemaking Topics; Micro-Oxygenation. Virginia Tech Wine/Enology Grape Chemistry Group, n.d.
(2) Singleton, V. L. Oxygen with Phenols and Related Reactions in Musts, Wines, and Model Systems: Observations and Practical Implications. 1987, 38 (1), 69–77.
(3) Waterhouse, A. L.; Laurie, V. F. Oxidation of Wine Phenolics: A Critical Evaluation and Hypotheses. American Journal of Enology and Viticulture 2006, 57 (3), 306–313.
(4) Gómez-Plaza, E.; Cano-López, M. A Review on Micro-Oxygenation of Red Wines: Claims, Benefits and the Underlying Chemistry. Food Chemistry 2011, 125 (4), 1131–1140.
(5) Fulcrand, H.; Dueñas, M.; Salas, E.; Cheynier, V. Phenolic Reactions during Winemaking and Aging. American Journal of Enology and Viticulture 2006, 57 (3), 289–297.
(6) McCord, J. Micro-Oxygenation: A Treatise. Stavin, 2009.
(7) Smith, C. Postmodern Winemaking: Rethinking the Modern Science of an Ancient Craft by Clark Smith (7-Jan-2014) Hardcover; University of California Press, 2014.
(8) Zoecklein, B. W. Current Theory and Applications: Microoxygenation. Practical Winery and Vineyard2007, November/December.
(9) Zoecklein, B. W. Factors Impacting Sulfur-like Odors in Wine and Winery Operations Part 2. Enolgoy Notes #134, 2007.
(10) Lesica, M.; Košmerl, T. Microoxygenation of Red Wines. Acta agricultura Slovenica 2009, 93 (3), 327–336.
(11) Gomez-Plaza, E.; Gil, R.; Lopez-Roca, J.; Adrian, M. Effects of the Time of SO2 Addition on Phenolic Compounds in Wine. Vitis -Geilweilerhof- 2001, 40, 47–48.
(12) Smith, C. MIcro-Oxygenation. Vinovation, n.d.
(13) Durner, D.; Ganss, S.; Fischer, U. Monitoring Oxygen Uptake and Consumption during Microoxygenation Treatments before and after Malolactic Fermentation. Am. J. Enol. Vitic. 2010, 61 (4), 465–473.
(14) Gambuti, A.; Han, G.; Peterson, A. L.; Waterhouse, A. L. Sulfur Dioxide and Glutathione Alter the Outcome of Microoxygenation. American Journal of Enology and Viticulture 2015, 66 (4), 411–423.
(15) Thomas, T. What’s in Wine: Acetaldehyde. Waterhouse Lab, UC Davis, 2004.