Due to the difficulties of the 2018 growing season, many winemakers had at least one or two lots in the winery with higher VA than desired. Unfortunately, once VA is present, there are limited options for remediation: masking with oak or sweetness, blending, and removal with reverse osmosis or nanofiltration technology. Following is a review of the principles behind VA removal. We begin with a general discussion of semipermeable membranes and why VA removal uses reverse osmosis. If you are familiar with these concepts already, you can skip to the second section outlining the specifics of reverse osmosis for VA removal.
Permeability and Concentration
Regardless of the type of filter being used, filtration itself depends on the principles of permeability and concentration. Filters act as semipermeable membranes, which provide a barrier that allows some particles to cross while impeding other particles. This is true for the filter pads used before bottling as much as the nanofilters found in the Sweetspotter machine. Filters can be constructed to sort particles based on a number of factors including size, charge, hydrophobicity, and others, however the filters we are concerned with sort primarily based on size.(1)
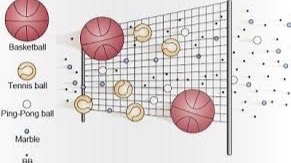
The size of molecules in solution are measured by units of molecular weight called the Dalton. If a membrane has a pore size of 100 Daltons, this means 50% of the molecules that are 100 Daltons are expected to pass through that membrane.(2) You can think of this in terms of making baskets with different sized balls. If you use a tennis ball in a regulation basketball hoop, rim shots are likely to go in, since the hoop is so much larger than the ball. If you try this with a basketball, the probability of the ball bouncing off the rim and out of the hoop is greater. If you try with a beachball, it will not go through. Many reverse osmosis filters are around 100 D. Given the molecular weights of common molecules found in wine (Table 1) we would expect water and ethanol to pass through this membrane easily, most of the acetic acid to pass, and some of the the ethyl acetate to pass through. When measured, it turns out that 75% of the ethanol, 60% of the acetic acid, and 40% of the ethyl acetate cross the membrane.(3)
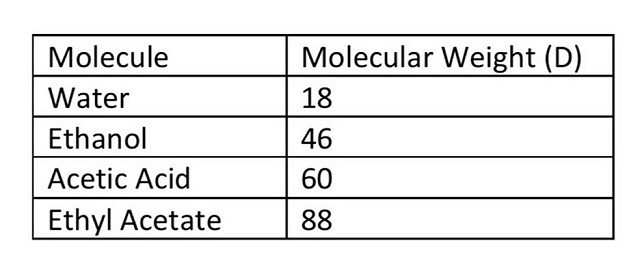
Table 1Molecular Weights of common wine chemicals. Adapted from Smith (2014)
The overall “size” of a particle really refers to its three dimensional structure. Proteins, for example, are long chains that fold in on themselves to form three dimensional shapes. This is analogous to a flat sheet of paper vs. a crumpled up sheet of paper. The membrane will sort based on whatever shape the molecule takes in the solution. Also, sometimes molecules interact with other molecules in ways that make them seem bigger to the membrane. Molecules in water based environments can even interact with the water itself. This is especially true for charged particles (ions), so any molecules in their ionized form will not pass as easily through the membrane (or may not pass at all). This many lead a membrane to retain particles that, based on weight alone, should be able to pass through (4).
VA removal is a membrane driven process that takes advantage of the permeability of membranes to separate out these small molecules of the wine while leaving the others intact. Most of the constituents of wine are much larger than the pore size of the membranes used for this process. By running the wine through the filter, water, ethanol, acetic acid and ethyl acetate are allowed through while tannins, anthocyanins, proteins, acids and most flavor molecules are retained.(2) The liquid that crosses the membrane is called permeate while the liquid that does not cross is called retentate. The retentate makes up the filtered wine while the permeate is either discarded or treated and returned to the wine, depending on the application.
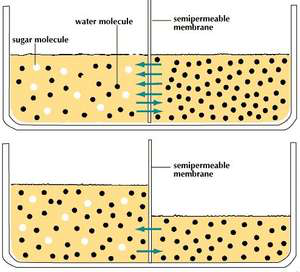
Figure 2 This membrane is permeable to water but not sugar. The water moves by osmosis from the side with less sugar (more water) to the side with more sugar (less water) to dilute the sugar solution.
The other principle that governs reverse osmosis is concentration. In solution, molecules naturally move by diffusion from areas of higher concentration to areas of lower concentration. The difference in concentration across a distance is called a concentration gradient. Molecules will only move in response to their own gradient, not the gradients of other molecules. For example if you have a chamber separated by a membrane that is permeable to both salt and sugar, and you add salt to one side of the membrane and sugar to the other, each will diffuse to the other side to even out the concentration. The presence of salt will not deter sugar from diffusing. (4)
Water also moves according to this principle. The diffusion of water is called osmosis. When thinking about osmotic movement it is important to remember that in any solution, the osmotic pressure represents the sum total of all of the chemicals dissolved in that water. If a solution is 5% sugar, it is 95% water. If it is 10% sugar, it is 90% water. So, if you have two chambers separated by a membrane that is permeable to water but not sugar, and you fill one chamber with 5% sugar solution and the other with 10% sugar solution, the water will diffuse from the 5% solution to the 10% solution by osmosis.
With that in mind, picture this: you have a membrane separating two chambers. The membrane pore size is 100 Daltons. If you start with wine on one side of that membrane, what will happen?
(1) Initially, water, ethanol, and acetic acid will cross the membrane while the remainder of the wine remains on the original side. There is no water, ethanol or acetic acid on the other side, so they are diffusing down their concentration gradients.
(2) Very shortly, there will be a solution of water, acetic acid and ethanol of equal concentration on both sides of the membrane.
(3) All net diffusion will quickly stop when the concentration of these three components is equal on both sides of the membrane. In reality, water will diffuse back to the other side, as there are more chemicals dissolved in the liquid on the original side, as all those phenolics, acids and flavor molecules are still there.
That is not a very efficient membrane filtration. To counter this movement of water (osmosis) back through the membrane, and to force even more ethanol and acetic acid through, a physical pressure is applied on the original (retentate) side of the membrane. This provides the force to push more solutes and water through the membrane. However, due to the pore size, only those solutes small enough to pass continue across.(4) This is the basis of reverse osmosis: a process by which a solvent passes through a porous membrane in the direction opposite to that for natural osmosis when subjected to a hydrostatic pressure greater than the osmotic pressure.
Reverse Osmosis for VA removal
The use of filtration for VA removal relies on the principles of permeability and concentration. Most are crossflow systems, using long parallel tubes to provide surface area and tangential flow to keep the membranes from getting clogged. As Jamie Goode explains it,
a portion of the wine is put through a tube under pressure, with walls made of a filtration membrane. Crossflow keeps the membrane from clogging, but requires a lot of surface area of membrane, so these systems use a column consisting of lots of small tubes. Water, acetic acid and alcohol pass through the filter to form permeate, after which it can be treated and returned to the wine. (5)
There are several different kinds of membranes used in cross flow filter systems with different separation applications depending on the type of filter. Table 2 gives an overview of pore sizes for membranes and what they are each used for. Reverse osmosis is the primary type used for VA removal.(1)
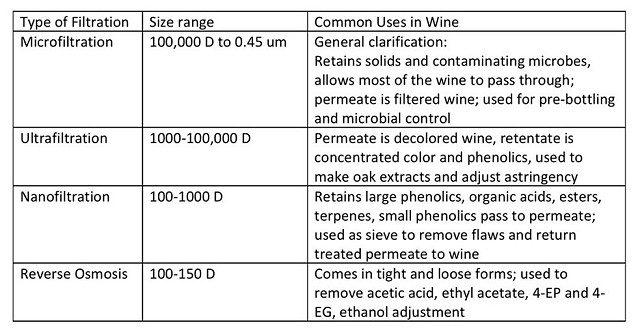
Table 2 Types of crossflow filters used in winemaking
The process of reverse osmosis can be applied at several steps in winemaking for different purposes. (5)
- Juice or must can be concentrated by the removal of water by RO. This technique has been used for decades in European countries with higher rainfall and lower Brix.
- Wine from California and other hot growing regions can be de-alcoholized when wine is run through RO, the permeate is distilled, and the water is returned to the wine.
- RO can also be used to concentrate the flavors of wine by running a small portion through RO and discarding the permeate (water, ethanol, acetic acid), thus concentrating phenolics, color, and flavor. Caution must be taken, as all of the flavors are concentrated, including methoxypyrazine and other off-flavors.
- Treatment of permeate after RO of wine can remove taints such as acetic acid, ethyl acetate, 4-EP, 4-EG, smoke taint and ladybug taint using ion selective columns to remove these molecules, after which the treated permeate is returned to the wine.
Despite relying on the same principles, machines outfitted for one purpose are not also optimized for other applications (must concentration and VA removal, for example) due to differences in pressure needed to overcome osmosis in juice vs. wine. Also, different membrane sizes may be needed.(6)
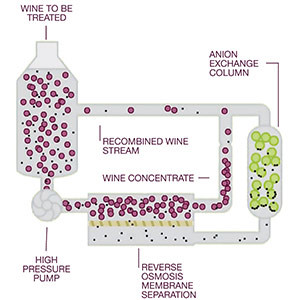
Figure 3 Steps to VA removal
To remove of VA specifically, three steps are needed. The Chateau Hetsakais website (7) outlines these fully:
(1) Run the wine though the RO filter to create a permeate of alcohol, acetic acid, and water while the leftover (retenate) is essentially the same wine with lower alcohol, less water and less acetic acid. For must concentration, one would stop here and discard the permeate.
(2) Reduce the pH of the permeate to less than 3.0 using a pH correction column. This shifts more of the acetic acid into its molecular form rather than its ionic form. The molecular form is the form that binds to the resin in the VA removal column in the next step, so the larger proportion of the acetic acid in this form, the better the binding efficiency will be.
(3) Once the pH has been shifted, the permeate is run through the acetic acid binding resin. The permeate increases in pH because as it binds to the resin, it displaces KOH, a base. After the permeate has traversed the column holding the acetic acid removal resin, the remaining permeate is closer to the starting pH and has much less acetic acid. This solution is then returned to the wine.
The process is similar for removal of ethyl acetate, however the pH shift is different due to the ionization of this molecule, and a different resin is used. Due to the importance of pH and availability of binding sites, preparation and regeneration of the columns is necessary, and pH must be monitored throughout the process. The Chateau Hatsakais website (insert hyperlink: http://chateauhetsakais.com/filtering-reverse-osmosis/) has a complete step by step guide of the practical steps involved in running one of these machines.
Despite this great technology, RO is often accompanied by flavor stripping and a shift in pH.(2, 5, 6) This is due in part to the type of membrane being used. Reverse osmosis membranes can be described as tight or loose. Tight membranes have smaller pore size and only allow water, ethanol, acetic acid and ethyl acetate to pass. However, these membranes also require higher pressure and energy requirements and have slower flow rates. Loose filters allow water, alcohol, acetic acid and ethyl acetate as well as 4-EP and 4-EG to pass (so they can be filtered out), but malic and tartaric acids can pass through the membrane as well, leading to large changes in pH that must be corrected later. These membranes have lower energy and pressure requirements and faster flow rates, so they are in common use. (6)
Smith (2017) advocates for only using tight membranes with MW cutoff of 80 Daltons. His argument is that, though these machines often come equipped with pH adjustment, if acids are making it through the membrane, most likely flavor molecules are too. Tight filters would retain acids and small sized flavor molecules. However, he points out that machines that use looser filters run at decreased pressure and increased flow rate. Smith acknowledges that machines such as the Sweetspotter by VA Filtration, though it uses loose RO membranes, is the “only alternative for micro-winery outside California (unless you have one of your own).” (This may have changed since Smith’s book.) These machines are outfitted for low energy operation that allows plug-in to 20 amp/110V wall outlet and can be used without refrigeration. He also compliments its “terrific instruction manual” and acknowledges that to substitute tighter membranes would lead to “impractically slow” permeate flow.
Each machine will be outfitted slightly differently, so if you are considering using this technology on your wine, make sure to contact the leasing company or manufacturer for specific instructions, and consider the pros and cons of this intervention. In his chapter on reverse osmosis, Jamie Goode (5) quotes Roger Boulton, renowned enologist from UC Davis, as cautioning there are “no independent published reports of the sensory effects of this treatment compared to a control, only proprietary claims and selective testimonials”. Different wines will respond differently to this procedure, but we will try to have examples of wines before and after treatment at several of the WRE tastings this year, in case you are considering this intervention yourself.
References
(1) Carey, R. (2017). Membrane Filtration - Wines and Vines (June 2017)
(2) Smith, C. (2014). Postmodern Winemaking: Rethinking the Modern Science of an Ancient Craft by Clark Smith. University of California Press
(3) Vinovation (n.d.) Uses of RO. Retrieved from http://www.vinovation.com/custequip.htm. Jan 1, 2019.
(4) Jackson, R. S. (2014). Wine Science: Principles and Applications(4 edition). Amsterdam: Academic Press.
(5) Goode, Jamie (2014). The Science of Wine: From Vine to Glass Second Edition. University of California Press. Berkely and Los Angeles.
(6) Smith, C. (2017). Selecting a Machine for Reverse Osmosis - Wines & Vines (Jan 2017)
(7) Filtering (reverse osmosis). http://chateauhetsakais.com/filtering-reverse-osmosis/. Retrieved 1/1/18.